Here’s how quantum mechanics lets heat cross a vacuum
New experiment shows that so-called quantum fluctuations allow heat to bridge tiny spans of empty space
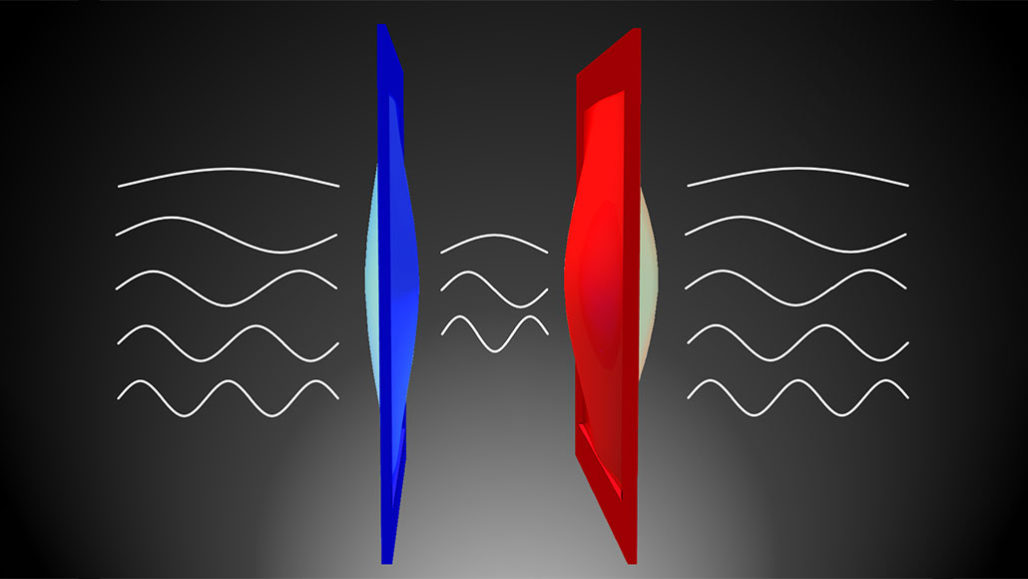
A weird effect of quantum physics can allow heat to cross a vacuum. An experiment has just shown this. Even in empty space, electromagnetic waves appear. They produce an attraction between two tiny, vibrating membranes (illustrated here in red and blue). This force equalized their temperatures.
Xiang Zhang/Univ. of California, Berkeley
Scientists have measured a new way to move heat across empty space. Such a transfer of heat had been predicted. It occurs thanks to quantum mechanics. That’s the physics theory that describes events at very small scales. Until now, however, this type of heat transfer had never been shown. In a new experiment, heat jumped across a tiny, empty gap just 300 nanometers wide (about one hundred-thousandth of an inch).
A vacuum would normally prevent most types of heat transfer. This helps explain why a vacuum-sealed thermos keep cocoa hot at a cold football game.
Heat typically travels through three main pathways: conduction, convection and radiation. Conduction describes heat transfer via direct contact of materials. Convection transfers heat through the motions of gases or liquids. (One example: Hot air rising.) Neither of those two work in empty space. But radiation — heat transfer via electromagnetic waves — can occur across a vacuum. In fact, that’s how the sun warms Earth.
Now “quantum mechanics gives you a new way for heat to go through” a vacuum, says King Yan Fong. This physicist worked on the study while at the University of California, Berkeley. But this heat transfer is noticeable only under special conditions. The span over which the heat moves must be amazingly small.
At nanometer distances, heat can cross a vacuum thanks to quantum fluctuations. Those are temporary particles and fields that appear for brief instants and then disappear. They occur even in empty space.
To test whether heat really travels this way, researchers set up an experiment. They used two tiny, vibrating membranes made of gold-coated silicon nitride. Each measured only some 300 micrometers (about a hundredth of an inch) wide. The researchers cooled one membrane and heated the other. They made one 25 degrees Celsius (45 degree Fahrenheit) warmer than the other.
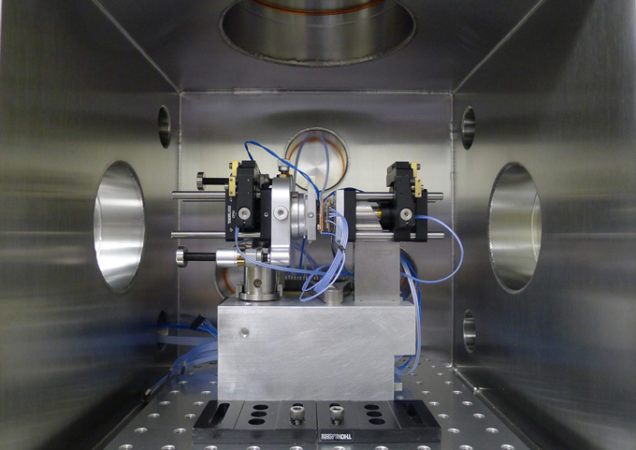
The heat caused the membranes to vibrate like the head of a drum. The warmer the membrane, the more vigorously it vibrated. Then the researchers moved the membranes to within about one hundred-thousandth of an inch of one another. Nothing separated them but empty space. Before long, their temperatures matched each other again. This showed that heat had moved between them.
The researchers shared their findings in the December 12, 2019 Nature.
“It’s super exciting,” says Sofia Ribeiro of Durham University in England, who was not involved with the study. She is a quantum optics researcher. She notes that scientists have been working to develop tiny machines that take advantage of heat at these quantum scales. The new study, she says, “opens … a huge platform that’s going to be very interesting to explore.”
What’s happening?
This new type of heat transfer results from what’s known as the Casimir effect. It describes how quantum fluctuations produce an attractive force between surfaces on either side of a vacuum in space.
According to quantum physics, empty space is never truly empty: Electromagnetic waves constantly blip in and out of existence. Although described as “virtual,” those waves can exert real forces on materials. In the vacuum between surfaces, those waves can only have certain wavelengths. But waves of any size can exist outside. And that excess of exterior waves can create an inward pressure. In the new experiment, the two membranes influenced one another by way of that force. The jiggling of the warmer object jolts the colder one, for example. That caused their temperatures to equalize.
“It’s a very neat experiment,” says physicist John Pendry. He works in England at Imperial College London.
This new type of heat transfer could be harnessed to improve how well nanoscale devices work. “Heat is a huge issue in nanotechnology,” Pendry says. How well the tiny circuits in cell phones and other electronics operate is limited by how fast the device can shed heat.
Pendry hopes to see future such experiments probe what role this effect might play in real-life devices. It would have been too much to ask for that in this first study, he says. That would be “greedy,” he admits.