Search speeds up for vaccine against the new coronavirus
Scientists are exploring unusual ways to make drugs to prevent or treat infections
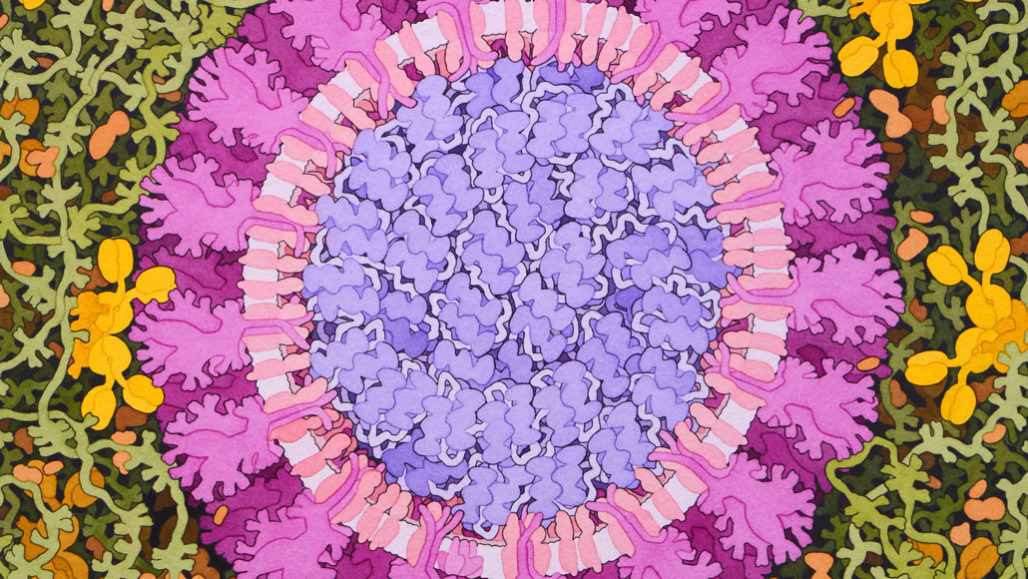
This artist’s false-color rendering depicts the virus that causes COVID-19. This new germ has inspired scientists to take creative approaches to making vaccines.
David S. Goodsell, RCSB Protein Data Bank
A mystery illness emerged in China late last December. As word of its spread got out, researchers at Inovio Pharmaceuticals paid close attention. This was even before anyone knew what was making people sick — a new coronavirus.
Inovio, based in San Diego, Calif., is no stranger to such viruses. A different novel coronavirus had emerged in 2012. It, too, caused potentially deadly infections. This disease would come to be known as MERS. (That’s short for Middle East respiratory syndrome.) Inovio became one of the first companies to develop a vaccine against MERS. (That vaccine is still experimental.)
Early in the latest outbreak, Chinese researchers posted details of the genetic makeup of the virus that was making so many people sick. The disease, which causes fevers, pneumonia and other serious symptoms, is now called COVID-19. The virus responsible has just been named SARS-CoV-2. Based on Inovio’s work on the MERS vaccine, its scientists sprang into action. They thought they might be able to roll out a version of the MERS drug to tackle COVID-19.
Kate Broderick is Inovio’s senior vice president for research and development. “We’d all hoped that there would be enough overlap that our previously developed MERS vaccine would be helpful in this case,” she recalls. Like MERS and another severe coronavirus — one that causes the disease SARS — the new virus uses RNA as its genetic material.
But in the end, Inovio’s researchers found, SARS-CoV-2 was too different for its vaccine against MERS to take down this virus. So the scientists set to work on creating a new vaccine.
Its design relies on a relatively new approach. Vaccines usually are made from weakened or killed forms of viruses or parts of viruses. Those viral parts may include some proteins that serve as building blocks of the germ. When injected into somebody new, their immune system recognizes these viral bits as an invader. It now makes antibodies. These should help to fight off future invasions by the whole, live virus. But to make enough vaccine for millions of people, it can take months or even years to grow enough disabled virus or to purify enough viral proteins.
So for their SARS-CoV-2 vaccine, Inovio scientists took a different approach. They converted the virus’s RNA into DNA. They also selected pieces of the virus that computer models suggested would prod the immune system into making antibodies. Then they inserted selected bits of the DNA into bacteria. Those bacteria now used instructions in the bits of DNA to make large quantities of the viral protein. And it’s those proteins that will be used in the vaccine.
This approach drastically shortens how long it takes to make a vaccine, Broderick says. Normally, it might take two to three years. For Inovio’s product, it took three hours to design. Then it took roughly a month to make, Broderick reports.
The company started testing the vaccine in animals at the beginning of this month. It hopes to begin safety tests in people by early summer.
Even so, Inovio’s vaccine is still at least a year away from widespread use. As the number of cases of COVID-19 continues to rise, several other research teams are also racing to develop vaccines and treatments. They, too, are using unusual ways to fight the new virus.
Novel vaccines for a novel coronavirus
Messenger RNAs are copies of protein-making instructions found in the DNA of genes. Cells “read” these instructions to build proteins. Researchers are now developing a messenger RNA — or mRNA — vaccine. Its goal would be to stimulate the body to produce vaccine components.
Part of the research team behind this project works for Moderna. It’s a Cambridge, Mass.–based biotechnology company. The other scientists work for the National Institute of Allergy and Infectious Diseases, or NIAID. It’s one of the National Institutes of Health.
Kizzmekia Corbett is an immunologist at NIAID’s Vaccine Research Center. It’s in Bethesda, Md. She’s also the scientific leader on the center’s effort to develop a COVID-19 vaccine. Scientists on this project have selected portions of the SARS-CoV-2 virus that may spark a vigorous immune reaction. This mRNA vaccine, she explains, would then tell human cells which viral proteins to make.
“We’re literally giving the cells a genetic code,” Corbett says. It’s being delivered as RNA. And it will tell cells, ‘Hey, make this protein.’”
Those proteins — Corbett wouldn’t say which ones — will then prod the immune system to make antibodies against the virus. Here, the body does all of the protein-making work. That means researchers can skip the time-consuming and costly step of making those proteins in some lab.
This approach could be used in making vaccines against future new coronaviruses or other new infectious diseases, Corbett says. “What we feel we have developed,” she adds, “is [a new way] to quickly deploy a vaccine if another novel coronavirus should pop up.”
Other mRNA vaccines for other infections are still undergoing tests.
On February 24, Moderna announced that the new mRNA vaccine is ready for testing in people. It will be tested first in 45 adults to see if it is safe. If it passes that test, researchers will do more testing to see if it also protects against the virus.
But even if the vaccine works, there is another problem. The researchers don’t yet have a company willing or able to produce the huge amounts of mRNA doses. And that would be needed to make enough vaccine to treat a large share of the public.
The long road to a new vaccine
Inovio’s work on its MERS vaccine shows just how long it can take to make sure a vaccine is safe and works as it should. The company ran safety tests of its MERS vaccine from February 2016 to May 2017. They recruited 75 healthy adults to take part in this phase I clinical trial. And there were no serious side effects, the researchers reported last September 1 in Lancet Infectious Diseases.
In August 2018, the vaccine moved into a Phase II trial. This tests a drug’s safety in a far more people. It also tests whether the vaccine spurs the immune system to make protective antibodies, as it was designed to do. That trial is expected to wrap up later this year.
Even if everything goes well, the MERS vaccine must still pass yet another clinical trial. This Phase III type investigates the drug’s safety and how well it works. Data from this type of trial must be submitted to the U.S. Food and Drug Administration, or FDA, before a company can release a new vaccine. All new vaccines and prescription drugs must pass such tests.
Inovio and the NIAID-Moderna team have both received funding from an organization in Oslo, Norway. It’s called CEPI. That’s short for Coalition for Epidemic Preparedness Innovations. CEPI also is funding work on yet another type of novel vaccine. CEPI and researchers at the University of Queensland in Brisbane, Australia, have found a way to keep a coronavirus from infecting cells. They refer to it as a molecular clamp.
The Queensland group had already been working with CEPI on such a “clamp” against other viruses for about a year, says Trent Munro. He’s a biotechnologist on the project. A molecular clamp, he explains is a protein stitched onto a second protein. In this case, it’s stitched onto the spike protein of a coronavirus.
With SARS and MERS, spike proteins work a bit like shape-shifting lock picks. They can change shape to interact with a protein on the surface of human cells. This allows them to enter those cells. On February 19, researchers described the 3-D structure of the spike protein of the new coronavirus in Science. This confirmed that new virus’s spike protein is also a shape-shifter. What’s more, it clings to its target on human cells 10 to 20 times as tightly as the SARS spike protein does to the same target. Such a tight grip may help the COVID-19 virus spread more easily from person to person, researchers say.
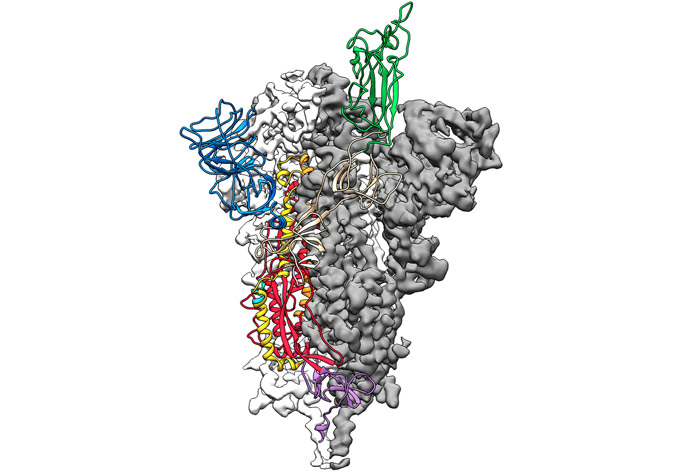
The molecular clamp that the Queensland team devised keeps the spike protein from shape-shifting. It locks it into a shape that triggers antibody production. This makes it a potent vaccine, Munro says.
When the Queensland group began working with CEPI to develop a molecular clamp vaccine, “we thought it would take three years as a test case,” Munro says. But the emergence of the new coronavirus forced the researchers to speed up their work.
The team is using mammal cells to make this vaccine. And a specialized machine figures out which cells are making the clamped protein. With this machine, Munro says, in just days researchers can “do things that would have taken weeks before.” Lab testing of the COVID-19 vaccine has already started. Testing in animals may begin soon. A safety test of it in people might be just months away. Still, Munro notes, it will be much longer than that — at least a year — before the vaccine is ready to roll out to the public.
“I know the timeline feels long,” Munro says. “I imagine it feels just unacceptable to those folks who are in areas of serious outbreak.” But he wants the public to know that they’re “pushing things forward as fast as possible.”
CEPI has plans to work with other groups on new vaccines. On January 31, it said it was partnering with a company in Tübingen, Germany. It’s for another mRNA vaccine to protect against the COVID-19 virus.
Beating vaccines to the punch
People who get over infections will retain antibodies to the germ that made them sick. It may stick around in their blood for years — even decades. Those antibodies can give them some protection when that person later encounters a similar germ. But those antibodies also might protect others.
How? Give people a shot of somebody else’s protective antibodies. This might prevent infections in healthy people. It might even treat infections in people who are already sick. And these injections could work faster than vaccines.
Vaccines can take weeks or months to prod the immune system into making enough antibodies to stave off an infection, says Christos Kyratsous. He works at at Regeneron Pharmaceuticals, based in Tarrytown, N.Y. There he is the vice president of infectious-disease research and technologies for fighting viruses. (Regeneron is a major financial supporter of Society for Science & the Public, which publishes Science News for Students.)
Ebola vaccines take at least a week to stimulate antibody production. In contrast, shots of “antibodies offer immediate protection,” Kyratsous says.
In studies by other research teams, blood serum taken from people who had recovered from Ebola helped infected people recover from the disease. And it did so because that serum was loaded with protective antibodies. Doctors and scientists in China have already begun using blood plasma from people who have recovered from COVID-19 to treat people who are sick with the disease.
One problem: Giving people antibodies from survivors doesn’t always work. Regeneron and other companies have developed antibodies that can offer more reliable protection. The company is already testing antibodies against Ebola and the MERS virus. Human trials and laboratory work with the company’s MERS antibodies suggest that these treatments can help protect against infection. They also can treat infections in people who are already sick, Kyratsous says.
Regeneron is now developing antibodies against the COVID-19 virus. “We have learned a lot of things from the MERS project that we can now apply to the novel coronavirus,” Kyratsous says.

For instance, the team has learned more about which parts of the virus make the best antibody targets. The proteins on the surface of a virus that are needed to infect cells — such as the spike protein — generally are best, he says.
Regeneron researchers have made SARS-CoV-2 proteins in the lab. They have injected them into mice that have human versions of antibody-producing genes. These “humanized” mice make human antibodies, Kyratsous says. Such mice could provide a ready supply of antibodies to treat people.
As soon as those antibodies are available, the company hopes to run lab tests on how well they work against the virus. If they work well, safety tests in animals and people might be able to start soon.
The team also hopes to get some blood from people who recovered from COVID-19 and retrieve some of their antibody-producing cells. But, Kyratsous cautions, mining antibodies from people’s blood won’t easily yield enough to treat masses of people.
As these programs all show, getting a treatment for a new virus is not something that can be done overnight. It can take months or years.
So in the midst of a new outbreak, “You’re not going to just pull a vaccine out of your pocket,” notes Anthony Fauci. He directs NIAID in Bethesda, Md. If the current outbreak proves to be “really bad,” the FDA might allow emergency use of promising vaccines that haven’t completed their full safety and efficacy testing. But researchers won’t know for at least six months whether any of the vaccines in development will help against COVID-19 virus.
Other strategies to fight the new virus, including using existing drugs designed to fight other diseases (such as AIDS and hepatitis C), also are underway. But no one knows which ones are winners. So for now, people exposed to the new virus must rely on their own immune systems and care from doctors and nurses to fight off COVID-19.







