Working up a sweat may one day power up a device
A new supercapacitor collects perspiration to power a sensor

New device could use the sweat from a good workout to charge up a supercapcitor. The energy it stories can then be used to power your phone or portable electronics.
portishead1/E+/Getty Images
One route to greener electronics may involve working up a good sweat. Engineers are designing energy-storage systems powered by perspiration. One uses the salty moisture to charge a supercapacitor (SU-per-ka-PASS-ih-tor). The energy it stores can later be used to light an LED or run some type of electronics.
Like a battery, a supercapacitor stores energy. Researchers described their new model May 11 in Advanced Materials. Such sweat-powered devices could pave the way to wearable tech that is both safer and more sustainable.
Today’s wearable electronics include gadgets strapped to the body, such as watches and fitness trackers. But engineers are also designing electronics that are part of clothing or stuck right onto the skin. “Many times they are made of materials that are not sustainable or eco-friendly,” says Ravinder Dahiya. He’s an electronics engineer at the University of Glasgow in Scotland.
Batteries power most wearable devices today. Those batteries often contain harmful chemicals, such as acids. When it’s time to dispose of batteries, their chemicals can harm the environment. For safer tech, “why not then use something which is [a] body fluid?” Dahiya asks.
Sweat serves as the electrolyte, or charge-carrying solution, for the new device. “That’s kind of a new way of using sweat,” observes Mallika Bariya. A materials scientist, she works at the University of California, Berkeley and did not take part in the new research. Electrolytes are an “important component of these supercapacitors or even batteries,” she notes. They’re needed for these devices to provide power.
People often think about sweat as gross or unwanted. But sweat is interesting, she argues. It can tell you about someone’s health. Also, its chemical makeup can vary depending on what part of the body makes it. This new work “really shows that sweat isn’t a … useless, icky fluid,” she says. “It’s something we should think about more.”
Perspire for power
To make their device, the Glasgow team started with a piece of cloth. It was made of polyester and cellulose, a tough material that forms cell walls in plants. On each side of the fabric, the researchers dropped a solution containing an electrically conductive polymer. Polymers are long molecules made up of chemical units that repeat over and over. Once the solution dried, the polymer layers became electrodes. Those electrodes store an electric charge.
Now you need sweat, which the cloth absorbs from the skin. Sweat contains salts. Each molecule of salt contains a pair of ions, atoms with an electrical charge. One ion is positively charged, the other negatively charged. In the supercapacitor, those charges part ways. Positive ions move to one electrode and negative ions onto the other. These ions react with the polymer.
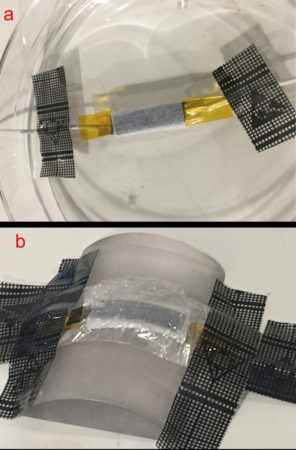
If the electrodes are connected to a device (such as an LED light), the electric current produced by the reactions can flow out to power it. This will eventually deplete the capacitor’s charge. Recharging it takes more sweat.
Some of the researchers strapped a capacitor onto their shirts and ran. The device was able to generate enough power to light up a few LEDs. The capacitor also powered a sensor that measured the saltiness of that person’s sweat. The team also tested the device with artificial sweat that included water and salts. The device now generated about five times more power than it had with the natural moisture. That may be because there hadn’t been enough sweat on the runners’ shirts to fully wet the supercapacitor.
The device has worked for thousands of cycles of charging and discharging. It kept its performance even when twisted and bent. It fared less well after washing. Some of the polymer washed away, causing a drop in the capacitor’s performance.
Sweat is “one of the few available energy resources on skin,” notes Seokheun Choi. An electrical engineer, he works at Binghamton University in New York. Using sweat, he points out, lets people harness energy that usually goes to waste.
The power these devices produce is quite low. But they might get a boost by teaming up with other systems that harness sweat, Choi says. He’s part of a team working on one such a device, a type of fuel cell. It relies on sweat-eating microbes to make energy. Such an approach could make exciting and sustainable electronics powered by perspiration.







