Moderna vaccine for COVID-19 appears nearly 95 percent effective
It’s a second leading candidate in a week to show preliminary success
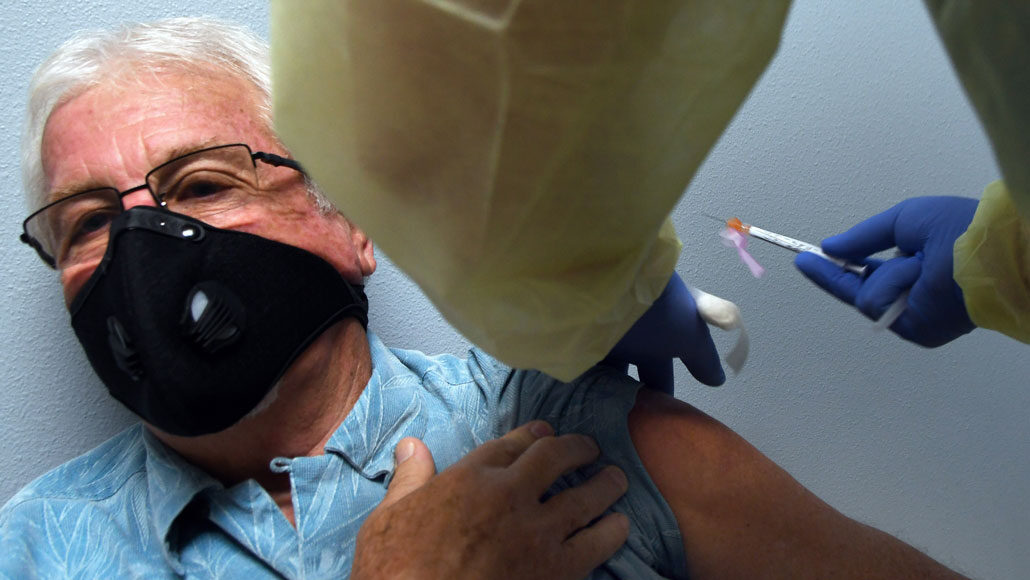
Tony Potts, here, is a 69-year-old participant in Moderna’s COVID-19 vaccine clinical trial. He received the company’s vaccine in August.
Paul Hennessy/NurPhoto/Getty Images
For the second week in a row, a major drug maker has issued data indicating that its new vaccine could be successful at fighting COVID-19. Preliminary results indicate that this coronavirus vaccine by Moderna Inc. is nearly 95 percent effective in preventing sickness.
These results are the “first clinical validation that our vaccine can prevent COVID-19 disease,” says Stéphane Bancel in a news release. Bancel is the company’s chief executive officer.
Moderna, based in Cambridge, Mass., developed the vaccine together with the U.S. National Institute of Allergy and Infectious Diseases. It’s in Bethesda, Md. Moderna, a biotechnology company, announced its promising data on November 16. This is an early release of the data. So far, those data have not yet been analyzed by outside scientists in a process known as peer review.
Only last week, global drug maker Pfizer and German biotech company BioNTech announced their coronavirus vaccine is more than 90 percent effective in keeping people from getting sick. If both vaccines continue to do well in clinical trials, the United States could soon have two vaccines available to treat those most at risk of coming down with COVID-19.
The U.S. Food and Drug Administration sometimes grants “emergency use” of drugs before they had passed through all of the normal steps required to approve new drugs. In the coming weeks, both Moderna and Pfizer plan to apply for this early use of their new vaccines.
Moderna’s results come from an analysis of 95 coronavirus cases. All had emerged among recruits taking part in its Phase III clinical trial of the vaccine. Researchers tallied who fell ill, but didn’t start this count until at least two weeks after someone had received their second shot. Of the people who got sick, 90 had received an inactive placebo. Only five had gotten the true vaccine.
Based on those data, the vaccine appears 94.5 percent effective. But those numbers could change because the trial is still ongoing. Moderna plans to follow participants for two years to monitor how safe its vaccine is and how long its protection lasts.
What we don’t know
The FDA recommends that COVID-19 vaccines be at least 50 percent effective. That means the treatment should reduce COVID-19 cases by half compared with no treatment (or a placebo). But how well a vaccine works can fall over time. Then a booster shot may be needed. Or the coronavirus that causes COVID-19 may morph genetically to where a revised version of the vaccine may be needed. So far, no one knows how well the vaccine will work over the course of a year or more.
It’s also not known whether the new vaccine works equally well in all ages or racial groups. The vaccine makers are trying to gauge that, however. The Moderna trial has included some older participants and people from different racial backgrounds. So far, of those who got sick, 15 of the 95 people were over age 65. Another 20 cases were Hispanic, Black, Asian or multiracial — all groups that have been especially hard-hit during the pandemic.
As of October 22, 30,000 people had enrolled in Moderna’s late-stage clinical trial. At that point, not all had been fully vaccinated. Thousands still needed to receive both shots, which are given one month apart.
The Moderna results do hint that people who get the vaccine may not get severely ill if they do become infected. So far, 11 people in the new trial got very, very sick with COVID-19. All were in the group that got a placebo.
It’s encouraging to see early results that show the vaccine can cut the risk of severe disease, says Nina Luning Prak. She’s an immunologist who works at the University of Pennsylvania in Philadelphia. Eleven is “still a small number,” she adds. “But it’s 11 out of 11 — versus zero on the other side.” And that’s what makes even that small number encouraging.
It’s also not clear whether the vaccine cuts risk of infection. Some people may get COVID-19 but just a mild case, one so mild that symptoms never emerge. And if that’s true for vaccinated people, can they still infect others? No one yet knows.
Side effects after the second injection of Moderna’s vaccine have been mild to moderate. Some people got tired, headachy or felt joint pain.
How the vaccine works
Both Moderna and Pfizer’s vaccines rely on messenger RNA, also known as mRNA. It’s a genetic molecule that a cell uses to “read” the instructions needed to build proteins. The mRNA in these vaccines contains instructions to build the spike protein (for which the coronavirus gets its name). That protein helps the virus enter human cells.
The vaccines allow our cells to make the spike protein. Our immune system can then then make antibodies to latch onto those spike proteins. Those antibodies may later prevent the real virus from infecting us.
To date, no vaccine using such mRNA technology has ever been used in people. “If such vaccines prove successful, this approach might speed up the vaccine-making process. That’s one benefit of the mRNA approach, says Luning Prak. “Within a matter of essentially minutes, you could basically design a vaccine.”
That’s because all researchers need is the genetic code for a specific protein in the virus that they are targeting. One example is that spike protein in coronaviruses. Other types of vaccines need cells grown in the lab to make millions of doses. Choosing the right viral protein to get the best immune response may still prove tricky, Luning Prak says. But mRNA vaccines “clearly have promise,” she believes.
One benefit of Moderna’s vaccine: It is not as heat-sensitive as the Pfizer-BioNTech drug. That vaccine must be kept frozen at an ultracold –70° Celsius (–94° Fahrenheit). That need to keep it super-cold can make moving Pfizer’s vaccine to where it will be used potentially difficult. Moderna’s vaccine, in contrast, remains stable for 30 days at refrigerated temperatures — between 2 and 8 °C (36 and 46 °F). That could help bring this vaccine to people in areas without easy access to dry ice or the specialized freezers needed for Pfizer’s vaccine.







