Explainer: What are chemical bonds?
The attractive forces that hold chemicals together drive the properties of each substance
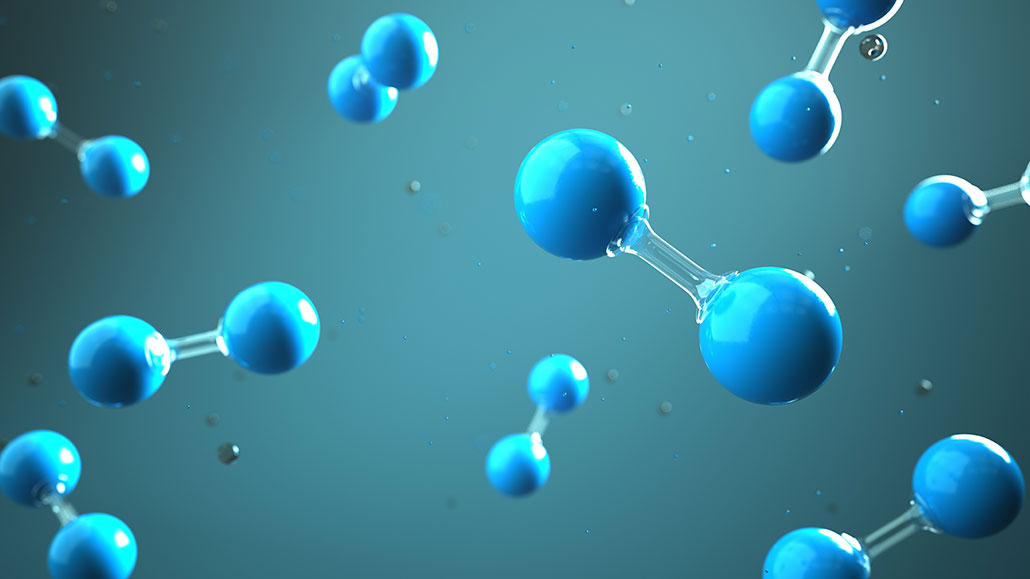
Here is an artist’s renderings of hydrogen molecules — pairs of hydrogen atoms held together by a chemical bond. This type, consisting of a shared pair of electrons, is known as a covalent bond.
style-photography/iStock/Getty Images Plus
Imagine a glass jar holding 118 types of building blocks. Every type is a slightly different color, size and shape. And each represents an atom of a different element on the periodic table. With enough jars, you can use the blocks to build anything — as long as you follow a few simple rules. A combination of blocks is a compound. Within the compound, bonds are what “glue” each of the blocks together. Additional, weaker types of bonds can attract one compound to another.
These bonds are quite important. Essential, really. Quite simply, they hold our universe together. They also determine the structure — and therefore the properties — of all substances. To know if a material dissolves in water, for instance, we look to its bonds. Those bonds also will determine if a substance conducts electricity. Can we use a material as a lubricant? Once again, check out its bonds.
Chemical bonds broadly fall into two categories. Those that hold one building block to another inside a compound are known as intra bonds. (Intra means within.) Those that attract one compound to another are known as inter bonds. (Inter means between.)
Intra- and inter-bonding are further divided into different types. But electrons control all bonds, no matter what type.
Electrons are of one the three primary sub-atomic particles that make up atoms. (Positively charged protons and electrically neutral neutrons are the others.) Electrons carry a negative charge. How they behave will control the properties of a bond. Atoms can give up electrons to a neighboring atom. Other times, they might jointly share the electrons with that neighbor. Or electrons can shift around inside a molecule. When the electrons move or shift, they create electrically positive and negative areas. Negative areas attract a positive area and vice versa.
Bonds are what we call those attractions between negative and positive areas.
Intra-bond type 1: Ionic
Electrons can be passed between atoms just like money can be handed from one person to another. The atoms of metallic elements tend to lose electrons easily. When that happens, they become positively charged. Non-metal atoms tend to gain the electrons that the metals lose. When this happens, the non-metals become negatively charged.
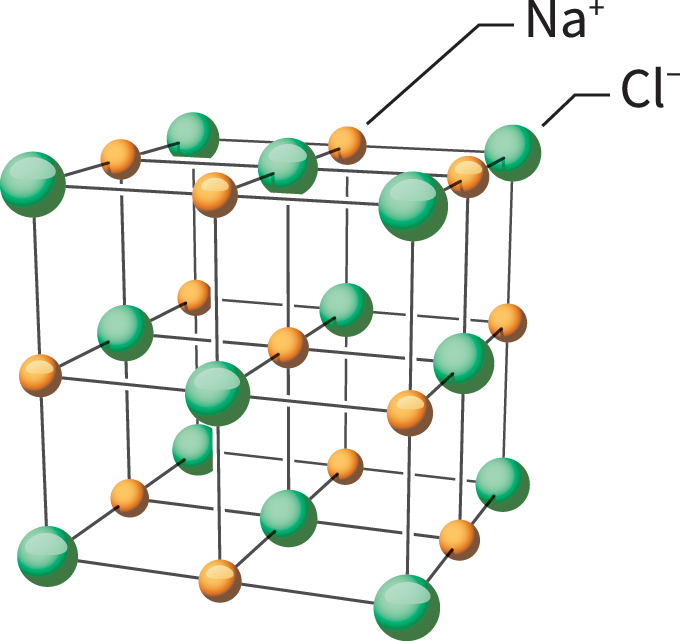
Such charged particles are known as ions. Opposite charges attract one another. The attraction of a positive ion to a negative ion forms an ionic (Eye-ON-ik) bond. The resulting substance is called an ionic compound.
An example of an ionic compound is sodium chloride, better known as table salt. Within it are positive sodium ions and negative chloride ions. All of the attractions between the ions are strong. A lot of energy is required to pull these ions apart. This trait means sodium chloride has a high melting point and a high boiling point. Those charges also mean that when salt is dissolved in water or melted, it becomes a good conductor of electricity.
One tiny grain of salt has billions and billions of these tiny ions attracted to one another in a giant, 3-D arrangement called a lattice. Just a few grams of salt could contain more than a septillion sodium and chloride ions. How big a number is that? It’s a quadrillion times a billion (or 1,000,000,000,000,000,000,000,000).
Intra-bond type 2: Covalent
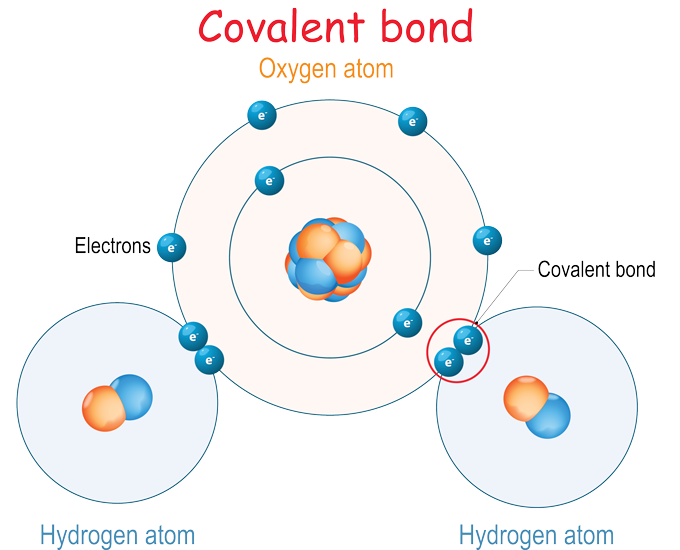
A second type of bond doesn’t transfer an electron from one atom to another. Instead, it shares two electrons. Such a shared pair of electrons is called a covalent (Koh-VAY-lunt) bond. Imagine a handshake between one hand (an electron) each from two people (atoms).
Water is an example of a compound formed by covalent bonds. Two hydrogen atoms each join up with an oxygen atom (H2O) and shake hands, or share two electrons. As long as the handshake holds, it glues the atoms together. Sometimes an atom will share more than one pair of electrons. In these cases, a double or triple bond forms. The small groups of atoms bonded together in this way are called molecules. H2O represents one molecule of water.
But why do bonds form?
Imagine standing on the very edge of the top step of a huge flight of stairs. You might feel unstable there. Now imagine standing at the bottom of the staircase. Much better. You feel more secure. This is why intra-bonds form. Whenever atoms can create a more energetically stable situation they do so. Forming one or more chemical bonds with other atoms gives the starting atom more stability.
Inter-bonding
Once covalent molecules form, inter-bonding can attract one molecule to another. Because these attractions are between molecules — never inside them — they are called intermolecular forces (IMFs). But first, a word about something related: electronegativity (Ee-LEK-troh-neg-ah-TIV-ih-tee).
This mouthful of a term refers to the ability of an atom within a covalent bond to attract electrons. Remember, a covalent bond is a shared pair of electrons. Imagine a molecule where atom A shares a pair of electrons with atom B. If B is more electronegative than A, then the electrons in its covalent bond will be shifted towards atom B. This gives B a tiny negative charge. We mark this using the lowercase Greek letter delta together with a minus sign (or δ-). The lowercase delta denotes a small or partial charge. Because negative electrons have moved away from atom A, the charge it develops is written δ+.
The shifting of electrons to create these positive and negative areas results in a separation of electrical charge. Chemists refer to this as a dipole (DY-pohl). As its name suggests, a dipole has two poles. One end is positive; the other negatively charged. The IMF is what develops between the positive pole of one molecule and the negative pole of another. Chemists call this a dipole-dipole attraction.
When hydrogen atoms bond covalently to very electronegative atoms, such as nitrogen, oxygen or fluorine, an especially large dipole develops. The intermolecular dipole attraction is the same as described above but is given a special name. It is called a hydrogen bond.
Electrons sometimes move around within bonds for reasons other than differences in electronegativity. For example, when one molecule approaches another one, the electrons within the covalent bonds of the two molecules repel one another. This creates the same type of δ+ and δ- charges as described above. And the same attractions occur between the δ+ and δ- parts. This type of IMF gets a different name: a London dispersion force.
No matter how electrons are moved to create the δ charges, the results are similar. Opposite δ+ and δ- charges attract to create IMFs between molecules.
Chemical changes, physical changes and bonds
Sometimes a chemical undergoes a phase change. Ice may melt into water or vaporize as steam. In such changes, the chemical — in this case, H2O — remains the same. It is still water: frozen water, liquid water or gaseous water. It’s the forces of attraction between the water molecules — the inter-bonds — that are broken.
Other times, chemicals may transform into a new substance. To get there, intra-bonds break and then new ones form. It’s like dismantling the building blocks from which you had made a racecar or a castle. Now you use their pieces to build a house or a table.







