Salt bends the rules of chemistry
At high temperatures and under high pressure, salt molecules reorganize in unexpected ways
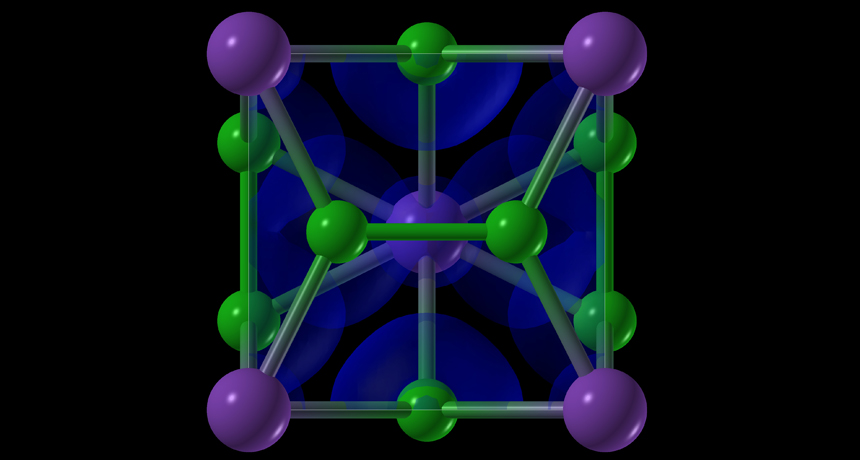
Under pressure and high temperatures, salt can form unusual structures. Here, purple dots represent sodium atoms and green dots represent chlorine. The blue areas represent electron clouds.
Oh salt, we thought you followed the rules. Now we find you sometimes break them — dramatically. Indeed, scientists have just used this cooking staple to bend the conventional rules of chemistry.
“This is a new chapter of chemistry,” Artem Oganov told Science News. A chemist at Stony Brook University in New York, Oganov worked on the salt study that shows some of chemistry’s rules are flexible. His team published its findings in the December 20 issue of Science.
Usually, the structure of table salt is orderly and neat. A salt molecule contains atoms of two elements: sodium and chlorine. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a single chlorine. Scientists used to believe this arrangement was a fundamental rule; that means no exceptions.
But now they find it was a rule waiting to be bent. Oganov’s team found a way to rearrange salt’s atoms using diamonds and lasers.
The salt was squeezed between two diamonds to put it under pressure. Then lasers aimed a powerful, focused beam of light on the salt to intensely heat it. Under these conditions, salt’s atoms linked up in new ways. Suddenly, a single sodium atom might attach to three chlorines — or even seven. Or two sodium atoms might link up with three chlorines. Those odd linkages change salt’s structure. Its atoms can now form exotic shapes never before seen in table salt. They also challenge the rules taught in chemistry classes about how atoms form molecules.
Oganov says the high temperature and pressure used by his team mimic the extreme conditions deep inside stars and planets. So the unexpected structures that popped out of the experiment might actually occur throughout the universe.
Scientists have long suspected that at high temperatures and pressures atoms might break the usual rules of how bonds form. In salt, for example, sodium atoms donate an electron (a negatively charged particle) to chlorine atoms. That’s because both the sodium and chlorine are ions, or atoms that have either too many or too few electrons. Sodium has an extra electron and chlorine wants it. This particle-sharing creates what chemists call an ionic bond.
In the past, scientists predicted that this electron swap would loosen up a little under high pressure and temperature. Instead of remaining fixed to one atom, electrons might move from atom to atom — forming what chemists call metallic bonds. That’s what happened in the salt tests. Those metallic bonds allowed sodium and chlorine atoms to share electrons in a new way. They no longer solely joined into one-on-one relationships.
Even though scientists expected the bonds could change, they weren’t certain. The new experiment now demonstrates that strange chemical forms can exist — even on Earth, Jordi Ibáñez Insa told Science News. A physicist at the Institute of Earth Sciences Jaume Almera in Barcelona, he did not work on the new study.
When the salt returns to low pressure and temperature, the novel bonds disappear, Eugene Gregoryanz told Science News. A physicist at the University of Edinburgh in Scotland, he also did not work on the study. Though the new finding is exciting, he said he would be more impressed to find metallic bonds in salt under less extreme conditions.
Indeed, he argues, if salt could hold such strange linkages under average conditions, that truly would be a “jaw-dropping discovery.”
Power Words
atom The basic unit of a chemical element.
bond (in chemistry) A semi-permanent attachment between atoms — or groups of atoms — in a molecule. It’s formed by an attractive force between the participating atoms. Once bonded, the atoms will work as a unit. To separate the component atoms, energy must be supplied to the molecule as heat or some other type of radiation.
electron A negatively charged particle; the carrier of electricity within solids.
ion An atom or molecule with an electric charge due to the loss or gain of one or more electrons.
laser A device that generates an intense beam of coherent light of a single color. Lasers are used in drilling and cutting, alignment and guidance, and in surgery.
molecule An electrically neutral group of atoms that represents the smallest possible amount of a chemical compound. Molecules can be made of single types of atoms or of different types. For example, the oxygen in the air is made of two oxygen atoms (O2); water is made of two hydrogen atoms and one oxygen atom (H2O).







