A new way to make plastics could keep them from littering the seas
For inspiration on how to make plastics break down, designers turned to RNA molecules

Due to plastic’s slow breakdown in seawater, 80 percent of trash in the sea consists of this long-lived synthetic material.
MaRabelo/iStock/Getty Images Plus
Chemists have spent the past century trying to make plastics that will break down in seawater. As it is, most plastics appear to take centuries to fully degrade in the ocean. That’s one reason plastics make up 80 percent of ocean trash. But that may change. Scientists have just designed a new plastic that can break down in seawater within weeks, not decades or more.
Back in the 1930s, scientists created a now-popular plastic out of corn and potato starch. It’s known as polylactide, or PLA. It’s a polymer, which is a molecule made by linking many building blocks — called monomers — into a long string. Scientists had hoped PLA would quickly break down in the environment. And in some places, like compost pits, it does. But not in seawater. Even after three years in ocean water, PLA remains largely unchanged.
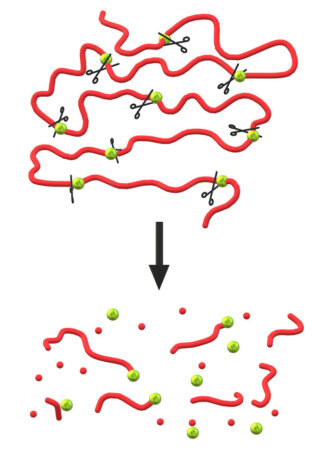
Timo Rheinberger is a PhD student at the University of Twente in the Netherlands. His work on polymers has focused on boosting PLA’s breakdown. As part of that work, he became part of a team that just added some biology-inspired breaking points to PLA. They put those breaking points to places where monomers in the PLA molecules are linked.
They weakened the links that joined up to 15 percent of a PLA’s monomers. Then, they soaked their samples in artificial seawater and measured how fast these tweaked versions of PLA broke down. The expected final product of PLA’s breakdown was a small molecule called lactic acid. So they tested for that too.

As the team had hoped, seawater attacked the weakened links between monomers, ripping the polymer chain apart. The more breaking points the researchers added to the polymer, the faster the PLA broke down.
When they weakened 15 percent of PLA’s monomer links, the polymer broke down entirely within just two weeks. When they weakened only 3 percent of the links, the breakdown took about 2 years. This suggests the team can design how quickly PLA will break down in water by adjusting how many weakened links it has.
The researchers shared their work October 4 in the Journal of the American Chemical Society. Says Rheinberger, this is “the first paper that looks at accelerating the breakdown of PLA in seawater on a molecular level.”
Borrowing from genetics
Think of a PLA molecule as a necklace of pearls, where each pearl is a monomer. Normally, those pearls stay firmly linked. That’s why normal PLA resists breaking down. But with the right chemical reaction, these pearls can separate.
Other chemists tried to rip apart PLA by swapping out some PLA monomers for other, weaker types. Chemists also tried blending pure PLA with other substances — materials that do degrade in the environment. Examples included plant-based fibers such as flax or hemp.
At first, that seemed to work. “The fibers get degraded,” Rheinberger says. “And the structure gets weak and falls apart.” But the PLA itself was not degrading, he adds. Its polymers remained intact.
That’s when his group decided to alter the links between monomers. Their inspiration for how to do this came from genetics.
All living things contain DNA and RNA. Together, these molecules help make and operate living things. DNA stores the instructions. RNA uses those instructions to make things. Like PLA, both are polymers. But they are made of different monomers.
As the ultimate instruction manual, DNA is made to last. RNA is not. Its molecules “have a short life,” says Rheinberger. Each RNA molecule is made for one job. When it’s done, the RNA breaks down, freeing its monomers for re-use.
Those monomers contain more groupings of oxygen and hydrogen atoms — called hydroxyls — than do DNA’s. That’s important. Those hydroxyls are where RNA’s breakdown starts. Hydroxyls attract water, making it easier for water molecules to attack an RNA molecule. More hydroxyls mean a faster breakdown.
Rheinberger and his team copied this hydroxyl-containing part of RNA and used it to link some of PLA’s monomers.
That hydroxyl-containing part of RNA is not an entire RNA monomer. Each RNA monomer has a five-sided “sugar ring” and a grouping of atoms called “phosphate.” To get the hydroxyl-containing piece, “we just take the phosphate and three bonds of the sugar ring,” Rheinberger says. The breaking points get their name from that phosphate. They’re called phosphoester (Foss-foh-ES-tur) linkages.
What comes next?
Mehlika Karamanlioglu teaches biomedical engineering at Istanbul Gelisim University. It’s in Turkey, just to the east of Italy and Bulgaria. She, too, has studied environmental breakdown of PLA. “It’s a new approach,” she says of the Dutch technique. Theirs is also “a preliminary study,” Karamanlioglu says. So more testing must follow. Scientists want to know how the strength of the new PLA compares to old PLA.
Rheinberger agrees. “You need a lot of material to start those studies,” he adds. And so far, his team has made only small amounts of the modified PLA.
Karamanlioglu notes the Dutch team also tested the breakdown of its PLA in artificial seawater. “I wonder if they checked [the water] for contamination,” she adds. If it was tainted with microbes, those microbes may have produced molecules called enzymes that sped up the PLA’s degradation.
Disposable cups and cutlery might one day be made from the new PLA. In theory, this new plastic could replace any old PLA, including the type used in many 3-D printers. And maybe scientists could add RNA-inspired breaking points to other plastic polymers as well.
Another place the new PLA might prove useful, Karamanlioglu says, is in biomedicine. PLA already is used to make the thread used to stitch up wounds. Sutures and medical implants must hydrolyze, meaning dissolve. “Sometimes you don’t want the implant to hydrolyze so quickly,” she notes. Perhaps RNA-inspired breaking points, she says, could provide a new tool for tailoring how quickly those medical implants break down.
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.







