Simple process destroys toxic and widespread ‘forever’ pollutants
In just hours, three ingredients can break down these formerly sturdy PFAS molecules

With the right equipment, scientists can find PFAS pollution in many places, including the water of many streams. And because these pollutants don’t naturally break down, they have come to be called “forever chemicals.” But they don’t have to be. A new process can break them down — and relatively quickly.
sinology/Moment/Getty Images Plus
By Nikk Ogasa and Janet Raloff
The solution to breaking down toxic “forever” chemicals may be found in your pantry. That’s the finding of a new study.
Since the 1960s, manufacturers have used fluorine-based chemicals in a range of household goods. These include nonstick cookware, oil-resistant food packaging, stain-resistant carpeting and water-repellent fabrics. Thousands of such synthetic compounds now exist. But while many of these didn’t let foods stick to your pans or water to penetrate your jacket, the chemicals do stick around in the environment. They just don’t degrade. In theory, they could persist for millennia.
This became a growing concern in 2005. That’s when data showed that trace levels of these chemicals taint the blood of nearly everyone in the United States. Soon, data would show that some of these compounds can be toxic. Adding to those concerns: These pollutants are showing up in water, and water-treatment plants have not been designed to remove them.
So the new study’s findings are welcome news.
These chemicals have long, technical names: perfluoroalkyl (Pur-flor-oh-AL-kul) and polyfluoroalkyl (Paahl-ee-FLOR-oh-AL-kul) substances. That’s why most people just call them PFAS.
The new study shows that three simple ingredients can quickly destroy PFAS. The first is ultraviolet light, or UV. It’s part of the spectrum of wavelengths found in sunlight. The second is iodide, a mineral often added to table salt. The third is sulfite, a common food preservative. Blasting UV light at a mix of PFAS, iodide and sulfite broke down nearly all the PFAS in a sample. It took mere hours.
Researchers shared their technique in the March 15 Environmental Science & Technology.
“It’s a big step forward,” says Garrett McKay. This environmental chemist, who works at Texas A&M University in College Station, did not take part in the new study.
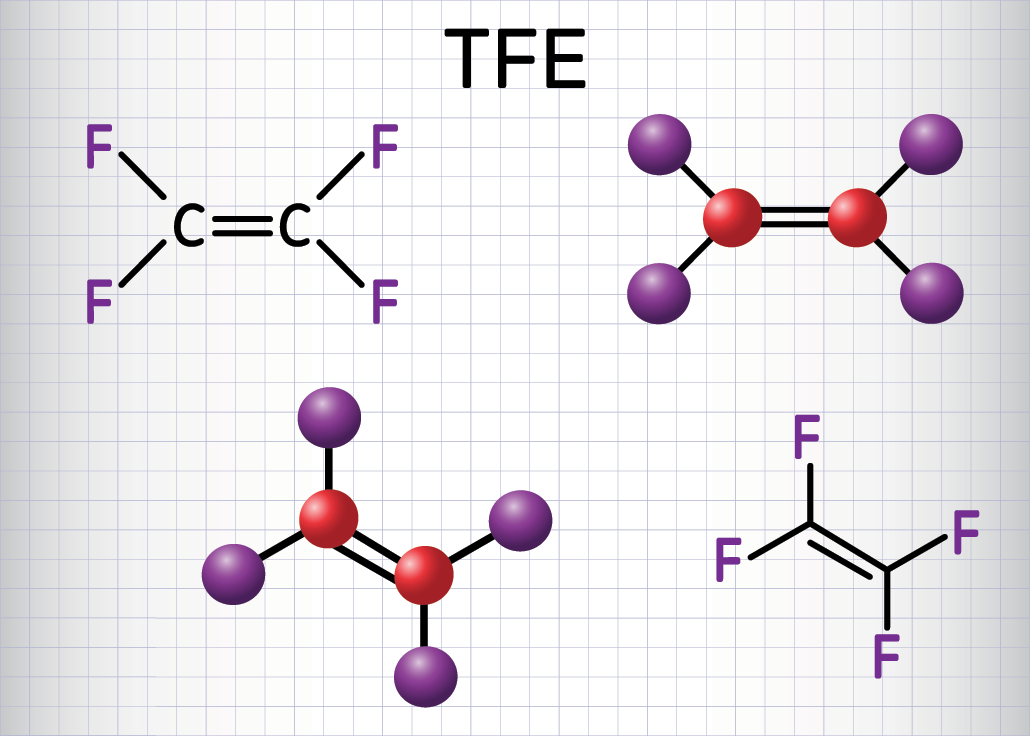
Why all the fuss over PFAS?
A PFAS molecule contains a chain of carbon atoms that are bonded to fluorine atoms. The carbon-fluorine chemical bond is one of the strongest known. This bond is what makes PFAS so useful for water- and oil-repellent coatings. PFAS also are used in firefighting foams and cosmetics.
But that sturdy carbon-fluorine bond has a downside. It prevents these compounds from breaking down in the environment. As a result, PFAS chemicals often pollute soils, food and drinking water.
Studies have linked high exposures to some of these pollutants to cancer, impaired immunity, heart disease and reproductive problems. One study out this May even linked these chemicals with reduced bone density in teen boys. Because most of an adult’s bone is produced during adolescence, “this finding may have implications for lifelong bone health,” says Abby F. Fleisch, one of that study’s authors. She works at the Maine Medical Center Research Institute in Portland.
On June 15, the Environmental Protection Agency issued new health advisories for four PFAS chemicals. “People on the front-lines of PFAS contamination have suffered for far too long,” said EPA Administrator Michael S. Regan. “That’s why EPA is taking aggressive action . . . to prevent these chemicals from entering the environment.”
EPA’s new advisories include two of the most common PFAS — PFOS and PFOA. EPA also, for the first time, issued a final health advisory for perfluorobutane sulfonic acid and its potassium salt (PFBS). PFBS is one of the PFAS compounds confirmed to effectively break down in the new study.
Picking apart PFAS
Some new water-treatment plants can remove PFAS using special filters. But those filters just collect PFAS. They don’t get rid of the chemicals. And those that aren’t destroyed risk sneaking back into the environment. So scientists have been exploring ways to get rid of PFAS wastes.
One of the most well-studied ways involves mixing PFAS wastes with sulfite, explains Jinyong Liu. He’s an environmental chemist at the University of California, Riverside. Liu is part of a team that has blasted that mix with UV rays. The UV light rips electrons from the sulfite. Those free electrons are highly reactive. They can snip the stubborn carbon-fluorine bonds in PFAS, he notes, creating smaller molecules.
But some PFAS have proven difficult to degrade this way. One example is PFBS. Such chemicals have been used to make fabrics, carpets and paper. Liu’s team blasted UV light at a mix of PFBS and sulfite for a whole day. By the end, less than half of the PFBS had broken down. To destroy more of it, the scientists had to shoot UV rays at the sample for even longer. They also had to keep adding more sulfite to the mix.
The iodide advantage
Liu’s group knew that when exposed to UV light, iodide produces more bond-cutting electrons than sulfite does. And past research had shown that together, UV radiation and iodide could degrade PFAS. The team suspected that adding iodide to their UV-sulfite mix would speed the breakdown of PFAS.
And it did. After a day of blasting UV rays at a mix of PFBS, iodide and sulfite, more than 99 percent of the PFAS was gone.
The process destroyed other types of PFAS similarly well. It could even degrade PFAS concentrations that were 10 times higher than could be broken down with UV light and sulfite alone.
Iodide and sulfite double-team the pollutant to destroy it, Liu explains. When UV rays release an electron from iodide, that iodide becomes a reactive molecule. Such a molecule then looks to grab freed electrons. Iodide could get in the way of electrons snipping up the PFAS. But sulfite is able to step in and bond with the reactive molecules. This helped keep the rogue electrons free to cut apart PFAS molecules for eight times longer than if sulfite wasn’t there.
It’s surprising that no one showed sulfite and iodide could team up to degrade PFAS before, McKay says.
Liu and his colleagues are now testing their process on PFAS in wastewater from a groundwater-treatment site. The pilot test will wrap up in about two years. Then, scientists will have a better sense of how well this technique works in the real world.







