Explainer: Radioactive dating helps solve mysteries
Knowing the decay rate of radioactive elements can help date ancient fossils and other artifacts

This Tyrannosaurus rex fossil can be dated — to know when it died (and therefore when it lived) — based on measuring the relative abundance of various radioactive isotopes in it and/or the surrounding rock.
Divaneth-Dias/iStock/Getty Images Plus
By Trisha Muro
You discover a fossilized bone and want to know how old it is. You can start by using the rock layers nearby to make a good guess about the fossil’s age. Maybe those clues tell you that the rocks are somewhere between 30,000 and 50,000 years old. That’s a big range. Fortunately, the science of radioactive dating can offer a more precise measuring tool for the bone itself.
The key is understanding the rate at which a radioactive element decays.
All the elements on the periodic table have isotopes. These are variations of an element’s usual form that contain the same number of protons but a different number of neutrons. Scientists know of 254 stable, non-radioactive isotopes. Some isotopes occur naturally. Others emerge only under special conditions in a lab. Some natural isotopes, and all lab-made isotopes, are unstable — they are radioactive. Forces within them are trying to jettison some extra mass (and energy). Eventually those forces win out. And this happens at a predictable, clock-like rate. That’s called the decay rate.
Knowing this decay rate allows scientists to look at something — like that fossilized bone — and gauge its age. They start by measuring the amounts of stable and radioactive forms of an element in the object. Then they compare how much of the original radioactive isotope has morphed into its decay products. Using math, scientists can then calculate how long ago that decay began. That’s the age of the object.
There are many elements that scientists can use in these sorts of studies. One of the most common is carbon.
All living tissues contain carbon. Most of that carbon is carbon-12. It has six protons and six neutrons. But a small share of that element will be carbon-14 — having eight neutrons. That form is radioactive. It’s known as a radioisotope. All living things contain roughly the same amount of this carbon in their tissues. Decaying carbon-14 is constantly replenished via the carbon cycle. Only once a creature dies will the share of carbon-14 in its remains begin to drop due to radioactive decay. That’s why measuring carbon-14 in a fossilized bone can show how long ago a creature died.
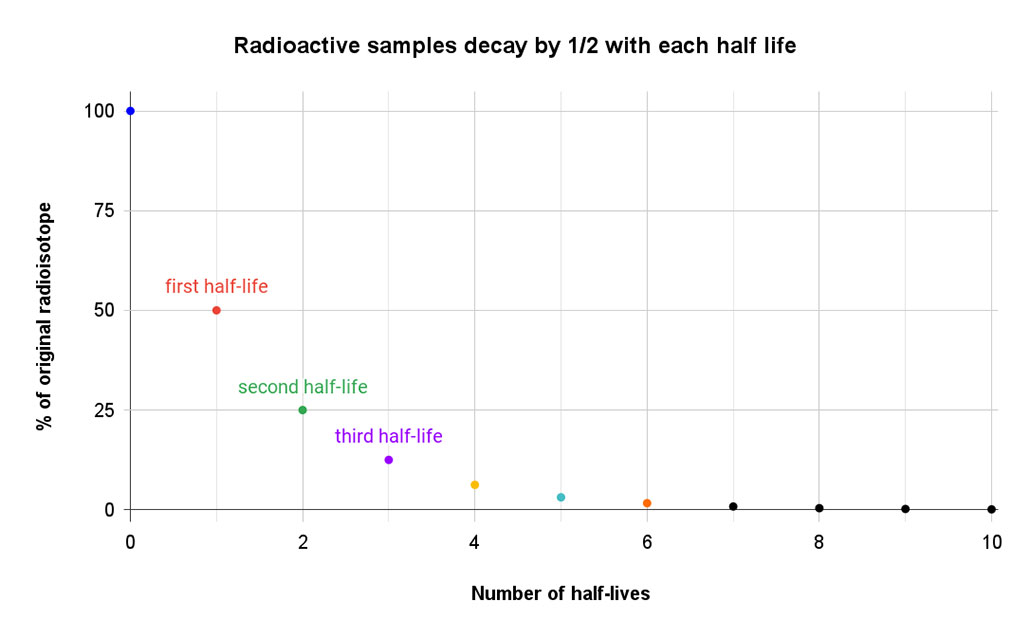
Carbon-14 has a half-life of 5,730 years. During each span of that time, half of this radioisotope in a bone will decay to nitrogen-14. That form of nitrogen (seven protons, seven neutrons) is stable and not radioactive. So the amount of the starting radioisotope drops by half in 5,730 years. After 11,460 years — two half-lives — it’s fallen to one-quarter of the starting amount. And every 5,730 years after that, the carbon-14 value will drop by half again.
Making good use of this decay
Bruce Buchholz works at Lawrence Livermore National Laboratory in California. A forensic chemist, he uses carbon-14 to solve mysteries, such as whether some piece of artwork is a forgery. He also helps with crime puzzles, such as when police need to know how long ago someone died. “The wonderful thing about using carbon-14,” he notes, “is that everything that’s living takes up carbon. It’s like everything is labeled.”
But carbon doesn’t work for dating everything forever. Scientists will choose a specific radioisotope as a yardstick for time, based on its half-life. (This is similar to how a carpenter might choose which screwdriver or chisel to pull from a toolbox based on the project for which it will be used.)
For instance, carbon-14 dating was used to determine that the cloth wrappings from a mummified bull in Egypt were about 2,050 years old. This matches other historical records from the pyramids. But to get the age of another sample from Africa that contained volcanic ash, researchers had to use a different element: potassium. Potassium-40 has a half-life of 1.2 billion years, which made it a much better option for dating the ash, which turned out to be 1.75 million years old. If the scientists had tried using carbon-14, they wouldn’t have found any. It would have all decayed and disappeared long ago.
Some radioisotopes are extremely rare or dangerous. That could make them impractical even if their half-life were a good match for the object being studied. Others, like carbon-14, are readily available and tell a clear story. It can show whether that fossilized bone you discovered is from a forest creature that died 800 years ago — and not some dinosaur that saw its end 80 million years ago.







