Explainer: All about carbon dioxide
It’s what makes our planet habitable — but there can be such a thing as too much

Carbon dioxide is naturally exhaled after every one of our breaths and is “inhaled” by plants for use in photosynthesis. This gas is a major component of our atmosphere — but too much there can trigger a warmer, more violent climate.
Oscar Wong/Moment/Getty Images
By Trisha Muro
Carbon dioxide, also known as CO2, is a simple molecule that makes life on Earth possible. CO2 is called a trace gas because it makes up less than 1 percent of the atmosphere. Even that small amount, however, plays a big role in shaping Earth’s climate. Without it warming Earth’s atmosphere, our planet would be too cold for most organisms to survive. But lately, this beneficial triplet of atoms has taken a lot of heat for making the atmosphere a bit too toasty.
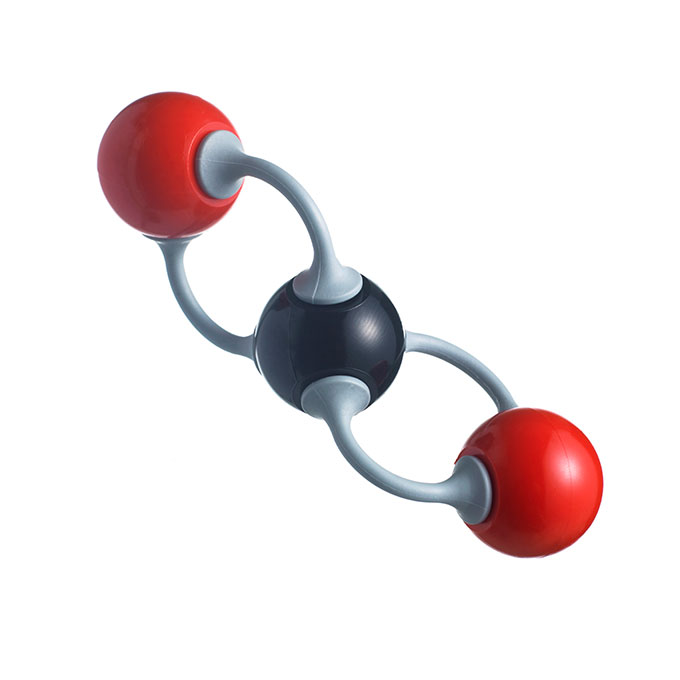
If you could zoom in to see a single molecule of CO2, it would look like a straight line with a carbon atom in the center and oxygen atoms at either end. One reason it forms this straight-line shape is its two double covalent (Koh-VAY-lunt) bonds. This means that the carbon shares two pairs of electrons — four electrons in all — with each of the oxygen atoms. These bonds are especially strong. (The CO2 molecule is also nonpolar, which means that all of the electric charges inside it are spread out relatively evenly.)
Mark Potosnak is an Earth scientist at DePaul University in Chicago, Ill. He describes CO2 molecules as being able to “flap their wings” a little bit. “Molecules with just two atoms, like oxygen (O2) and nitrogen (N2), really can’t wiggle much,” he says. “But with three atoms all in a line, CO2 can wiggle the oxygens at each end.” This helps the molecule absorb heat when hit by the sun’s rays.
At typical conditions on Earth, CO2 is colorless and odorless. But cool it to -78° Celsius (-109° Fahrenheit) and it turns solid. This “dry ice” looks snowy white and can transform directly from a solid to gas. If you’ve ever walked through a haunted house with a so-called “smoke” machine, the fog is likely vapor from a block of dry ice. (Always be careful around dry ice! It can cause frostbite quickly.)
CO2 can be captured in high-pressure canisters and used to inflate bicycle tires or power paintball guns. It’s also the source of the fizz in carbonated drinks.
CO2 is heavier than air, which means it will sink. If CO2 builds up rapidly, such as at the bottom of a tunnel or in an enclosed room, this gas will push oxygen out of its way. In some cases, this can cause a person — or other animal — to suffocate. But since fire cannot burn without oxygen, CO2 is used in some fire extinguishers as a quick, effective way to douse flames.
Educators and Parents, Sign Up for The Cheat Sheet
Weekly updates to help you use Science News Explores in the learning environment
Thank you for signing up!
There was a problem signing you up.
A warm, life-sustaining jacket
Animals — be they humans, whales, turtles, fish or insects — “exhale” CO2 when they breathe. (Many microbes do, too.) Plants “breathe in” this CO2 and use it for photosynthesis. So do seaweeds and algae. This greenery then “exhales” oxygen, releasing it back for animals and other organisms to use. In this way, life on Earth recycles oxygen and CO2.
Besides living organisms, other natural sources of this gas include volcanic eruptions and fires. Over the eons, CO2 began accumulating in Earth’s atmosphere. It’s still there in only trace amounts. But a little goes a long way. It helps warm Earth’s surface through what’s known as the greenhouse effect.
Just like the glass walls of a greenhouse trap heat inside a building’s structure, CO2 acts like a blanket to hold heat close to our planet’s surface. Earth would be largely uninhabitable without it. (The James Webb Space Telescope has discovered evidence of CO2 on an exoplanet. Perhaps the gas helps warm that distant planet as well.)
Mars, by contrast, is very cold — partly because it’s not massive enough to hold onto much of an atmosphere. Thus, Mars simply can’t hold much of the energy it receives from the sun.
For much of Earth’s history, that greenhouse effect has helped the planet maintain temperatures that support life. But there can be too much of a good thing.
In recent years, global CO2 levels in Earth’s atmosphere have risen dramatically — to roughly 415 parts per million. (By January 15, 2023, it was 420.45 ppm at a monitoring station atop Hawaii’s Mauna Loa observatory.) Like a thick blanket, this growing excess traps more heat. And it’s now much higher than most ecosystems have evolved to find comfortable. Instead of feeling warmed on a cool night by a cozy blanket, many organisms now feel as though they’re lying under a pile of thick blankets in the middle of summer.

Runaway greenhouse warming
In recent decades, Earth’s greenhouse effect has shown signs of being out of control. That’s long been an issue on Venus. Its thick, dense atmosphere has led to surface temperatures that average a blistering 453° Celsius (847° Fahrenheit). That’s hot enough to melt lead. Despite its proximity to the sun, the surface of Venus would be far cooler if the planet had only a thin, Earth-like atmosphere.
For much of history, Potosnak says, levels of CO2 in the air have risen and fallen naturally. There has been a relative balance between the sources that spewed it and the processes that removed it. But things have changed. Burning fossil fuels, such as coal, oil and gas, releases lots of CO2. Those fuels power our cars, electronics and more. “Now,” Potosnak says, “we’re amping up that natural [CO2 release] process so fast that it’s throwing our systems out of whack.”
Increases in CO2 levels that “should take thousands of years,” he says, are developing within decades. That’s shown by the dramatic and seemingly skyrocketing “Keeling curve.” This graph of CO2 levels shows them climbing steadily over the past several decades. (The curve gets its name from scientist Charles David Keeling, who collected a lot of the early data.)
Indeed, from 1990 through 2021, “the warming effect on our climate … by long-lived greenhouse gases rose by nearly 50 percent, with CO2 accounting for about 80 percent of this increase.” The World Meteorological Organization, part of the United Nations, issued those findings on October 26, 2022. A year earlier, it pointed out, CO2 concentrations had reached 415.7 parts per million in air. That’s 49 percent higher than before the Industrial Revolution, when use of fossil fuels started to really take off.
It’s now known that humanity’s use of fossil fuels has changed the balance of our ecosystems. That’s causing “a whole cascade of problems,” says Potosnak. “What should take thousands of years, we’re doing within decades by burning so much fossil fuels.”







