Sea life may suffer as plastic bits alter metals in water
The interplay between plastics and metals could change how each affects the environment

Tiny, broken fragments of plastic trash (like that seen here) drift through seawater. Such plastic makes up the majority of ocean litter.
pcess609/iStock/Getty Images Plus
Once it gets into the environment, plastic trash tends to break into increasingly tinier pieces. These fractured bits have been winding up on mountain tops, in the oceans and everywhere in between. But these micro- and nano-bits of plastic don’t just collect like inert bits of sand or dirt (which is how researchers had tended to think about them). They may interact with other materials in the environment, new data show.
When exposed to light, bits of plastic in water can react with metals, such as manganese. And that, a new study finds, could spell trouble for hungry sea life.
Young-Shin Jun is an environmental engineer. Her team at Washington University in St. Louis, Mo., has shown that sunlight turns bits of plastic into micro-factories. Those factories pump out mobs of ions, which are charged particles. These particular ions contain oxygen and are known as reactive oxygen species, or ROS.
Oxygen is a double-edged sword. We need it to stay alive. But it’s wickedly reactive. “Oxygen species are nasty,” notes Kenneth Nealson. He’s a biogeochemist at the University of Southern California in Los Angeles. Reactive oxygen can harm cells, he notes. Think of ROS as oxygen’s dark side. Too much sunlight can damage our skin, for instance, through its production of ROS.
Lots of plastic ends up in the sea. There’s plenty of metal dissolved in seawater, too. ROS ions carry a negative charge. Dissolved metals make positively charged ions. The metal ions can join up with negatively charged particles to form salt-like crystals. So Jun’s team was interested in how the dissolved metals in seawater might interact with ROS from plastic.

The researchers focused on the metal manganese. (The plum-colored sands of Pfeiffer Beach in California get their hue from manganese-containing minerals.) The team mixed nanoplastic beads with dissolved manganese. After putting the samples under bright light, they watched what happened.
As expected, the plastic created ROS. But what happened next was a surprise: The dissolved metal ions buddied up with ROS and became solid manganese crystals. “Any heavy metal — iron, chromium, arsenic or whatever” could do the same, Jun suspects. Her team shared its unexpected finding in the November 28 issue of ACS Nano.
These new data suggest that interactions between metals and plastics — especially in the ocean — could be important. “Without thinking about the reactivity of nanoplastics,” Jun says, we might “overpredict or under-predict” plastic’s impact on the environment.
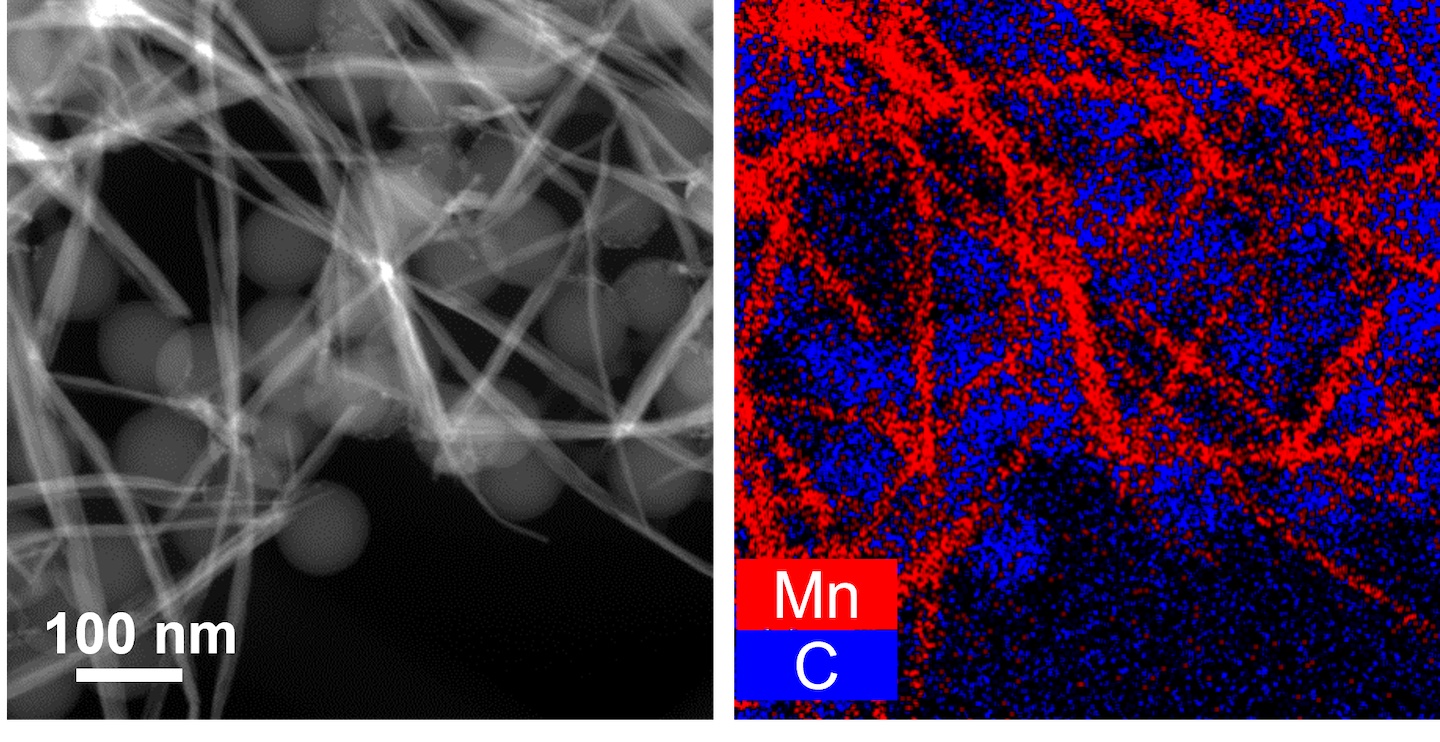
A ‘furry’ coating
The metal crystals that form can cloak the tiny plastic bits. That cloak gives these bits unexpected properties. Manganese-coated beads became “a furry nanoplastic,” Jun says. That fur, she now worries, could be cause for concern.
Dissolved metals act very differently than solid ones. If plastic trash causes metal to transform in water, might this affect fish, oysters and other ocean life?
Dušan Palić calls it “a highly likely possibility” that chemical reactions triggered by plastics could threaten the health of ocean life. A fish veterinarian, Palić works at Ludwig-Maximilians University Munich in Germany. Although he wasn’t involved in the new work, he does study what happens to animals and fish that eat nanoplastics.
The tiny bits of plastic start smooth, Palić notes — until ROS ions force the manganese to solidify. “Now you have needles essentially protruding out” from the plastic bits. What’s more, these furry nano bits clump together. Big clumps might look like food to some animals. For instance, zooplankton may try to dine on metal-spiked morsels. Trying to eat the spiky bits could kill them.
Some metals are also very reactive chemically. Palić wonders whether their reactions might damage an animal’s tissues, such as the fragile underside of gills. And if other metals similarly react with plastic, that could increase the risks. Fish might ingest solid chromium crystals, for example, thinking they’re food. In stomach acid, those crystals could dissolve. That would release dissolved chromium, which is toxic to fish.

A hidden opportunity?
The metallic fur that forms on nanoplastic bits could be bad for sea life but an aid to controlling the spread of this pollution. Or at least that’s a possibility, says Nealson at USC.
Unlike smooth nanoplastics, clumped furry bits tend to settle to the bottom. That would pull them out of the water. And that could offer a sort of opportunity, he says: “If you had a really polluted place with plastic, why not throw in … manganese?” It’s cheap, he notes. “Everybody’s worried about ROS.” But manganese would remove the ROS as it reacts to form fur. Once furry clumps sink to the seafloor, he says, they should be less likely to cause problems.
Nature already uses this manganese trick for cleaning up ROS, Nealson notes. He points to radiation-resistant bacteria. “We find them in the desert,” he says, where they endure long bouts of intense sunlight that would kill most microbes. One way these bacteria “fight this is by filling their cells up with manganese,” he says. It works because the “manganese interacts with the ROS before the ROS can destroy [their] proteins.”

Educators and Parents, Sign Up for The Cheat Sheet
Weekly updates to help you use Science News Explores in the learning environment
Thank you for signing up!
There was a problem signing you up.
Overall, Nealson is impressed. “Every bit of science has to start with showing that something can happen,” he says. “And that’s what they did,” he says of Jun’s group.
He now asks, why not use manganese to sop up the ROS from plastic? Although not without risk, he thinks it’s worth investigating. In this early study, Nealson notes, manganese levels were about “a thousand times more concentrated” than in a typical lake. The light levels were also high — perhaps four times higher than a typical day at noon. And the water’s pH could have a major effect on what happens to manganese in these situations. So it will be important to see what happens under real-world conditions.
Until now, Jun says, studies have mostly focused on the physical effects of plastic trash breaking down into polluting bits. They’ve largely overlooked possible chemical changes to the plastic. And that, she argues, is what we should be looking at next.







