Nanocrystal ‘painted’ films may someday help relieve summer heat
Unlike A/C, these colorful coatings might chill homes and cars without electricity

Peacock feathers get their bright blues and greens not from colored pigments, but from microscopic structures that refract and reflect light. Thin nanocrystal films use a similar strategy to showcase vibrant hues without warming in sunlight.
sarayut Thaneerat/Moment/Getty Images Plus
As summer heats up, future homes may cool down, thanks to coatings of vibrantly colored nanocrystals.
Normal coatings, such as paint, heat up in the sun. The new crystal films get cooler than the air around them when exposed to sunlight. They do this by reflecting the sun’s rays and releasing that heat into outer space.
Those coatings could provide a sustainable way to keep cool — no power required — for cars, homes and offices. This would be a big step up from air conditioners, which guzzle a lot of energy and can leak gases that add to global warming.
Qingchen Shen and his colleagues created a colorful selection of the new coatings. Shen studies materials science at the University of Cambridge in England. His team shared its work on March 26 at the spring meeting of the American Chemical Society (ACS). It took place in Indianapolis, Ind.

Unusual cooling
Surfaces that get cooler than their surroundings are unusual.
The reason: Hot objects shed heat through invisible infrared light. They transfer their heat to the air around them. When the object and the air reach the same temperature, that transfer stops. So the cooling stops.
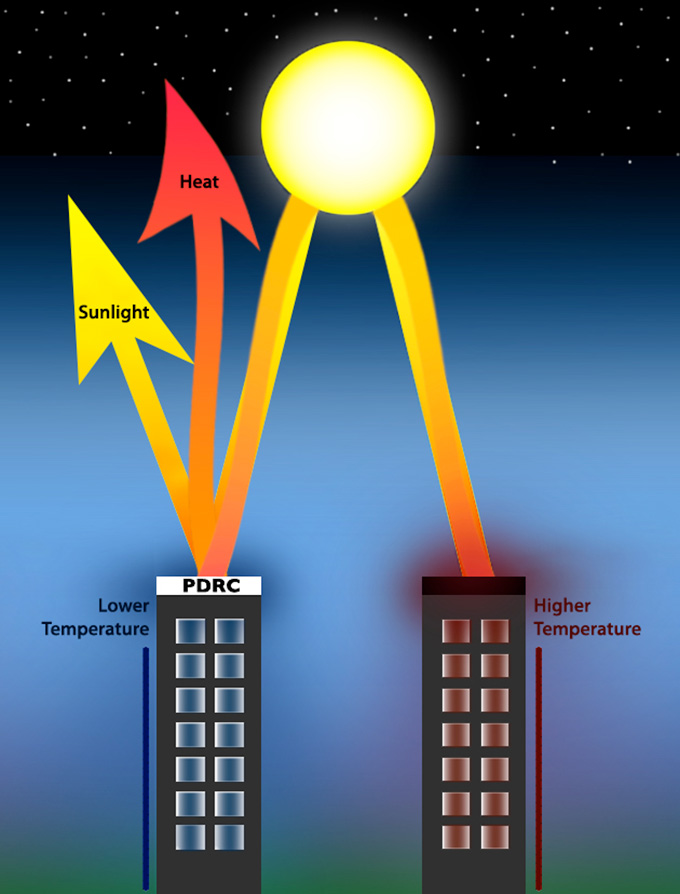
This explain why, say, a sun-warmed car hood emits heat that warms the air inside and outside the car.
But certain wavelengths of infrared light aren’t absorbed by air. They can escape the atmosphere into space. The new films release heat at these wavelengths. So they can give off heat without warming the surrounding air — and get cooler than the air around them, even when bathed in sunlight.
This cooling method has a long name: passive daytime radiative cooling. Shen’s team didn’t invent the process. Other materials do it too. But typically, there’s a trade-off: Until now, only white or mirrored surfaces did this. Such surfaces reflect a lot of light, keeping that light from heating the surface.
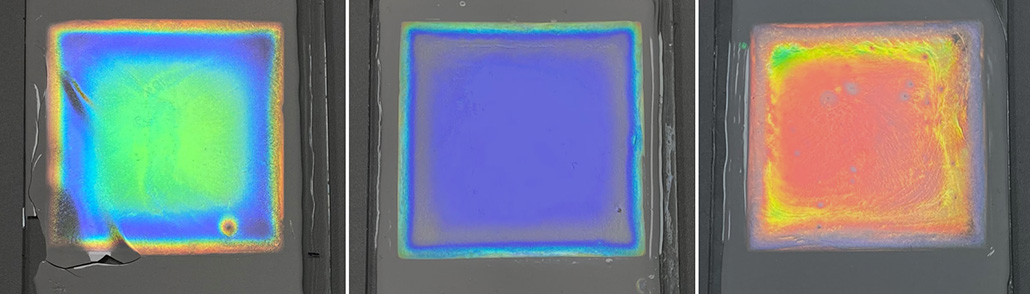
Now, Shen’s team has figured out a way to make colorful passive-cooling surfaces. They include vibrant shades of red, green and blue. But what he’s really proud of are the various textures. The team has created everything from glittery iridescent films to a calming natural-wood grain.
Color from crystals
The new films don’t get their color from pigments, which give paints and clothing their hues. Those chemicals work by reflecting just the colors of light we see and absorbing the rest. That absorbed light heats the material.
That’s why “if you wear a black T-shirt, you become warmer than if you wear a white T-shirt,” explains Silvia Vignolini. She’s a chemist at the University of Cambridge who also worked on the new films.
If those films got their color from pigments, they would absorb some of the sunlight, says Shen. That would heat them up and counteract the cooling effect.
Instead, the films’ color comes from microscopic structures. This is known as structural color. Tiny patterns on the films’ surfaces don’t absorb light. Instead, light waves bounce off them in particular ways, such that only certain colors reach our eyes. Changing the microstructural patterns changes what color we see.

Structural color is common in nature. The rainbow sheen of a soap bubble comes from structural color. A peacock’s vibrant feathers are another example. So are some baboons’ bright faces, Vignolini says. Their cool blue patches come from tiny beads of a protein called collagen.
The new films’ color comes from tiny crystals of cellulose. They are made from plant fibers. Cellulose isn’t just abundant and eco-friendly. It also emits heat as those infrared wavelengths that can escape the atmosphere.
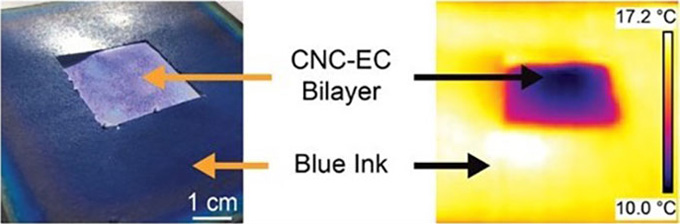
The film has two layers. The top one is crystallized cellulose, which provides the color. Different crystal patterns produce different colors. A different form of cellulose — ethyl cellulose — makes up the bottom layer. This layer is pitted and scatters all the light that leaks through the top layer.
Think of light particles as pinballs, says Lucian Lucia. “They hit the surface, and they bounce — boom, boom, boom … like a pinball in the machine.” Lucia studies biomaterials at North Carolina State University in Raleigh. He wasn’t part of this study.
Both cellulose layers help the film release heat that zips up into space. The two-layer approach is what makes this work unique, Lucia says. The Cambridge team, he says, achieved “a fairly remarkable and simple approach towards cooling.”
The team made the films one layer at a time. First, they dried a watery mix of ethyl cellulose into thin sheets. Then they spread a thin layer of cellulose crystals in water atop this, like icing a cake. As that “icing” dried, its crystals linked up to form the colorful top layer. Slight variations in crystal shape will yield different patterns — and colors.
Under sunlight, the films cooled to 4 degrees Celsius (7 degrees Fahrenheit) below the air temperature. At night, the temperature difference was 9 degrees C (16 degrees F). Shen’s team debuted its findings before the ACS meeting in Advanced Science.
Even better, Shen’s team worked out how to make the films in large amounts, which is “very important for their work,” notes says Ran Zheng. He studies materials science at the University of California, Los Angeles. Such large-scale production makes this innovation more likely to get used in real life, he explains. If used to help keep buildings cool in the summer sun, he notes, these coatings might cut the need for air conditioning.
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.
Correction: This story was updated on November 22, 2023, to clarify Qingchen Shen‘s descriptions of the films.