Experiment: Make your own cents-able battery
Make your own ‘voltaic pile’ with pennies and nickels, and find out how many coins will make the most electricity

Did you know that you can make your own battery by stacking up spare change? Let’s see how many coins generate the most electricity!
Meindert van der Haven/iStock/Getty Images Plus
Objective: Make a simple battery out of coins and test if the number of coins in the pile will affect the amount of electricity produced.
Areas of science: Energy & Power
Difficulty: Easy beginner
Time required: ≤ 1 day
Prerequisites: To do this project, you will need an adult to help you use a multimeter. Science Buddies’ How to Use a Multimeter guide will teach you how to use one.
Material availability: Readily available
Cost: Very Low (under $20)
Safety: No safety issues
Credits: Sabine De Brabandere, Ph.D., and Sara Agee, Ph.D., Science Buddies
You might think that batteries are a modern invention, but batteries were one of the first ways of making electricity. Alessandro Volta discovered the first electric battery in 1800. He made a giant stack of alternating layers of zinc, blotting paper soaked in salt water, and silver. This early design for a battery became known as the voltaic pile.
How does a voltaic pile make electricity? The key to electricity is the movement of particles carrying electric charge. In a voltaic pile, these particles move from one metal to the other through a solution called the electrolyte. An electrolyte is a liquid that contains particles carrying charge. Dissolved salt is an example of a good electrolyte.
The charged particles in the electrolyte react with the metals, causing an electrochemical reaction, a special kind of chemical reaction that makes electrons. Because electrons are particles that carry electric charge, making these electrons all move in the same direction will create an electric current, or electricity. You can read more about the basics of electricity in the Science Buddies Electricity, Magnetism, & Electromagnetism Tutorial.
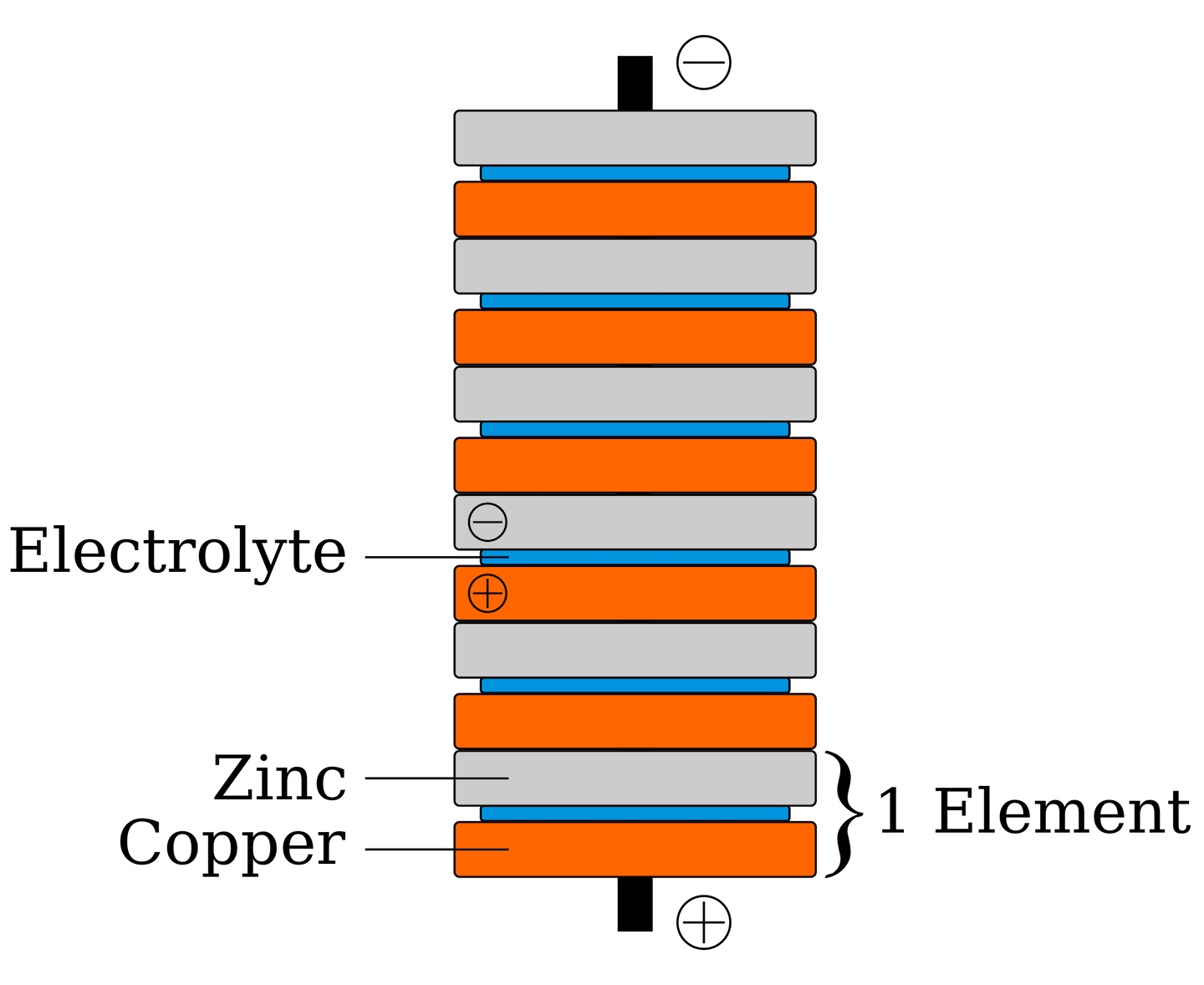
The two types of metals in a voltaic pile are called electrodes. The types of metal are different: one metal will like to give off free electrons, the other will be more eager to receive electrons. This creates an electrical potential difference, also called voltage, between the two types of metals. One metal becomes positively charged (the positive electrode), and the other becomes negatively charged (the negative electrode). This voltage causes electrons to move, creating an electrical current, and then you have electricity!
In this experiment, you will make your own version of the voltaic pile using two different types of coins (two different kinds of metal) and a salt-vinegar solution (the electrolyte). The metal in the coins will react with the electrolyte. Because the two metals are different, one metal will like to give electrons to the other, creating electricity. How will different numbers of coins affect the amount of electricity produced? To test this, you will make piles with different numbers of coins and measure the voltage (measured in volts) and current (measured in amperes) produced.
Terms and Concepts
To do this type of experiment, you should know what the following terms mean. Have an adult help you search the internet or take you to your local library to find out more!
- Electric charge
- Electrolyte
- Electrochemical reaction
- Electrons
- Current
- Electrode
- Voltage
Questions
- What materials can a battery be made out of?
- Why is it important for the materials to be arranged in alternating layers?
- What does the electrolyte solution do?
- Will more layers make a more or less powerful battery?
Materials and Equipment
- Vinegar (any kind, 1/4 cup)
- Salt (1 Tbsp.)
- Small bowl or glass
- Pennies (4)
- Nickels (4)
- Dish soap
- Aluminum foil (small strip)
- Scissors
- Lab notebook
- Paper towels (2) or construction paper
- Small plate (ceramic, plastic or Styrofoam — not paper or metal)
- Digital multimeter. See our multimeter tutorial if you do not know how to use a multimeter.
Experimental Procedure
1. In your lab notebook, make a table like Table 1. You will write down your measurements in this table.
| Number of pennies | Number of nickels | Voltage | Current |
2. In a small bowl or glass, mix together 1/4 cup of vinegar (electrolyte) and 1 tablespoon of salt (ions). Stir well.
3. Gather some pennies and nickels, wash with a mild detergent (like dish soap) and dry. This is just a preliminary step to remove dirt and grime.
4. Using scissors, cut a strip of aluminum foil, 2 centimeters by 8 centimeters (0.8 inch by 3.1 inches). Fold lengthwise in thirds as shown in Figure 2. Aluminum foil is a good electrical conductor. It will help create good electrical contact with the bottom penny of your pile.

5. Using scissors, cut up a paper towel or piece of construction paper into small squares, each a little over 2 by 2 centimeters (0.8 by 0.8 inch). You can trim the corners to make circles that are slightly smaller than the pennies. This will prevent the corners from dripping over the edges of the coins, which can cause a short circuit in your battery.
6. Place a dry paper towel on a plate as shown in Figure 3. You now have all the materials to start building.

7. Place the aluminum strip in the middle of your plate. You will build your battery on top. This strip will make it easier to connect the multimeter later.
8. Start building your stack:
- Put down a penny on the aluminum foil.
- Soak a paper towel or construction paper circle in the vinegar-salt solution. The paper piece should be wet throughout but not dripping.
- Place the vinegar-soaked paper layer on top of the penny as shown in Figure 4.
- Add a nickel on top of the paper piece, as shown in Figure 5. This is a tiny battery. You will add 2 more coins before measuring.
- To add more coins, put down a penny on the top nickel.
- Repeat steps 8.2 to 8.4. You now have a stack of four coins (alternating pennies, wet paper towel pieces and nickels), ending with a nickel on top.
- Important: Do not let the paper layers droop over the edges of the coins and touch each other. This will create a short circuit and prevent your battery from working. If necessary, use scissors to trim the corners of the paper layers so they do not hang down and touch the paper towel below them.
- Important: Paper layers should be wet but not dripping. Dripping electrolyte can create a short circuit. If necessary, press out excess liquid from the paper layers by placing them between your thumb and a finger.


9. Make a measurement. See the Science Buddies reference How to Use a Multimeter if you need help.
- Connect the probes (also called leads) of the multimeter to the two ends of the battery by placing one probe tip on the aluminum foil strip at the bottom of the stack and the other on the nickel at the top of the stack. Figure 6 shows the setup.
- Measure the voltage produced by your battery: Set the multimeter to measure voltage as shown in Figure 6. Push down on the multimeter probe tips to make good electrical contact. Write down the measured value (number only, not the sign) in the table like Table 1. Positive or negative measured values are both fine; you are interested in the number value without the sign. Consult the Frequently Asked Questions for more advice.
- Measure the current produced by your battery: Set the multimeter to measure current and record the current in your data table right away (the current may begin to drop slightly as the battery begins to drain). The multimeter setting to measure current is shown in Figure 7. Consult the Frequently Asked Questions for more advice.
- Note: You might encounter a negative reading. The sign informs you about the direction of the current. You do not need to pay attention to the sign for this project. You are interested in the magnitude (number).


10. Add another penny, soaked paper layer and nickel to the stack and measure again. As you add to your stack, one important rule is to always start with a penny and end in a nickel, so the numbers of penny layers and nickel layers will always match. Why do you think this is necessary?
11. Repeat step 9 for your new stack. Do not forget to record the number of pennies, the number of nickels and the measured voltage and current in your data table.
12. Repeat steps 10 and 11 one more time. You now have a stack containing 4 pennies and 4 nickels, like the stack shown in Figure 8.

13. You can keep repeating steps 10 and 11, building batteries consisting of more and more coins.
14. Analyze your data.
- Your data table is now complete. Can you observe a trend?
- Making graphs may help you visualize your data. If you need help creating graphs, try the Create a Graph website.
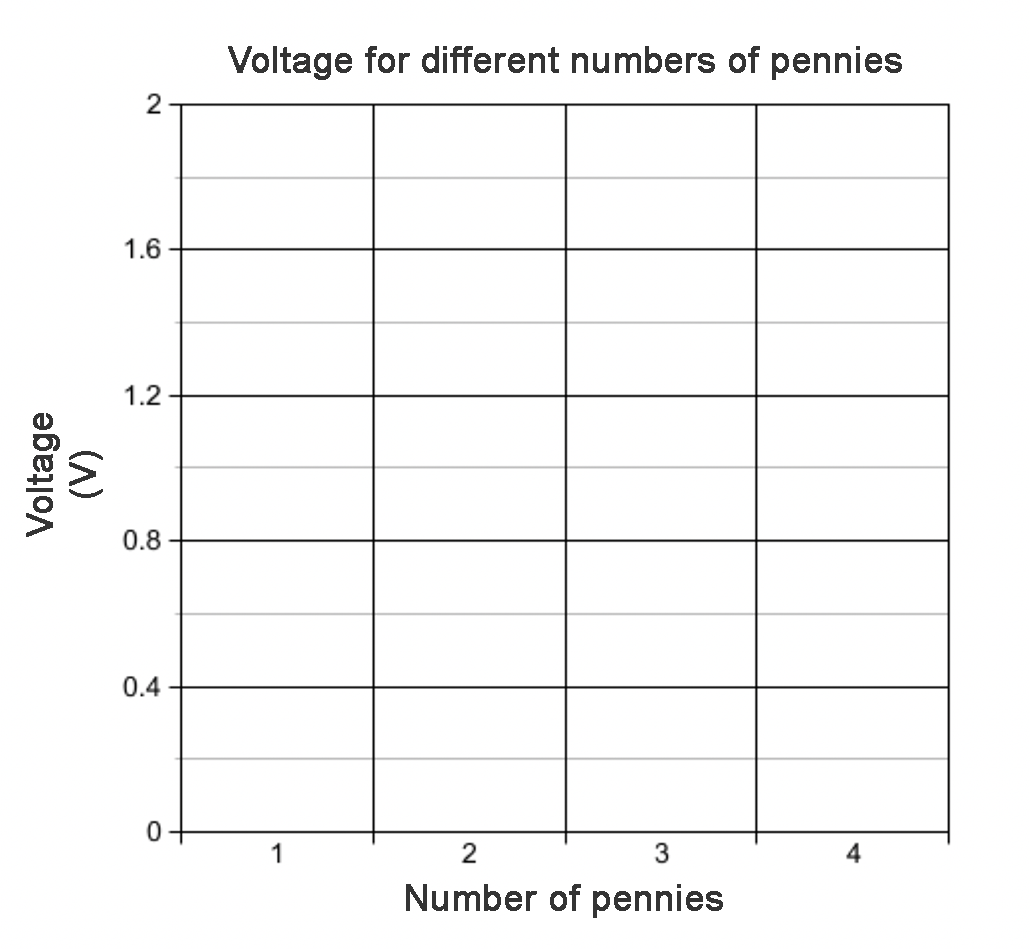
- Make a bar graph of the voltage (Y-axis) versus the number of pennies (X-axis). Do not forget to label the axes and add a title. An example is shown in Figure 9. Your graphs will show all your measurements.
- Make a bar graph of the current (Y-axis) versus the number of pennies (X-axis).
- How do voltage and current change when you add more coins? Are your results consistent with what you expected?
15. Repeat the entire experiment (Steps 2 to 14) twice more. Start all over again building a new battery from pennies and nickels. Scientists always perform several measurements to confirm their results. Do you get the same measurements each time? Do you see the same trend?
For troubleshooting tips, please read FAQ: A Battery That Makes Cents.
Variations
- Try connecting an LED to your battery with copper wire or aluminum paper strips. How many coins do you need to light the light? You can test different LEDs to see if they need the same number of coins to light up. (LEDs only pass current in one direction, so be sure you have it oriented correctly.) Note on copper wire: Be sure the wire is NOT enameled, or it will not work! Enameled copper wire can be used if you first strip the insulation off. This can be done with sandpaper, as explained in this Wire Stripping Tutorial.
- Compare different coin combinations to see which ones work and which ones don’t:
- Penny – Dime
- Nickel – Dime
- Nickel – Quarter
- Penny – Quarter
- Try other electrolyte solutions to see which ones work and which ones don’t:
- Plain water
- Salt water
- Lemon juice
- Soda water
- Try making batteries out of other things, like potatoes or fruits. Try the Science Buddies experiment How to Turn a Potato Into a Battery for more ideas!
This activity is brought to you in partnership with Science Buddies. Find the original activity on the Science Buddies website.








