Predicting and designing protein structures wins a 2024 Nobel Prize
A biochemist and two computer scientists using AI shared the top award in chemistry

David Baker (left) figured out how to build new proteins. Demis Hassabis (middle) and John Jumper (right) developed an AI tool to predict protein structures.
Niklas Elmehed, © Nobel Prize Outreach
By Meghan Rosen and Andrea Tamayo
For their work to unlock the mysteries of those building blocks of life known as proteins, three scientists received the 2024 Nobel Prize in chemistry.
David Baker won for his research on understanding and decoding the structure of proteins. Demis Hassabis and John Jumper won for their work in how to predict a protein structure. The Royal Swedish Academy of Sciences announced its recognition of the trio’s achievements October 9 at a news conference in Stockholm.
Proteins enable nearly every facet of life. They build our bones, skin and hair. They carry cargo from cell to cell. Some are antibodies that target dangerous microbes. Some are molecular machines that fix damaged DNA. Their list of roles in the body is nearly endless.
Each protein is made of a string of molecules called amino acids. Fold the string up, and you’ve got a protein.
But how the string folds is key. It can pleat like a paper fan. It can bend into spirals. It can crumple like a piece of paper — or do something else entirely. There’s a universe of potential shapes. Each depend on the order of amino acids in its string.
And just like the shape of a key determines which lock it can open, the shape of a protein influences its role in the body.
“To understand how proteins work, you need to know what they look like,” Johan Åqvist said. He’s a member of the Nobel Committee for Chemistry. “That’s what this year’s laureates have done,” he explained during the press conference.
Decoding a protein structure
Baker is a biochemist at the University of Washington in Seattle. In 1998, he was part of a team that debuted a computer program called Rosetta. It could take a short amino acid sequence and predict what the resulting protein’s 3-D structure would look like.
But a real breakthrough came in 2003. That’s when Baker’s team flipped that idea around. They sketched out a made-up 3-D protein — one that did not exist in nature — and asked Rosetta to come up with a string of amino acids that would fold into that shape.
And it worked.
Do you have a science question? We can help!
Submit your question here, and we might answer it an upcoming issue of Science News Explores
When Baker’s team synthesized the amino acid sequence in the lab, it folded into a protein just like the one Rosetta had predicted. Since then, Baker has created a trove of designer proteins. These include one that can sense the deadly opioid fentanyl in the environment and another that blocks the SARS-CoV-2 coronavirus.
“David Baker opened up a completely new world of protein structures that we had never seen before,” Åqvist noted. “It’s almost as if only your imagination sets the limit for what you can do.”
Leslie Vosshall has used Rosetta in her own lab at Rockefeller University in New York City. “These software algorithms have accelerated the ability of all scientists to do their work,” this neuroscientist says. “What used to take decades or forever can now take a minute.” Vosshall is also vice president of the Howard Hughes Medical Institute (which has been funding Baker’s work).
Predicting how a protein will fold
Hassabis and Jumper work at Google’s DeepMind in London, England. In 2016, both of these computer scientists had already gained worldwide fame for creating a computer program that beat the reigning world champion — a human — at the strategy game Go.
Two years later, the pair used their artificial intelligence chops to solve an even trickier problem. The duo’s AI model, called AlphaFold, could predict protein structures from amino acid sequences with almost 60 percent accuracy. That far surpassed anything done before.
Clearly, that was already a step forward, Åqvist said. But a second version of the model, AlphaFold2, was even better. It worked nearly as well as the gold-standard method for figuring out protein structures. That’s a lab technique called X-ray crystallography.
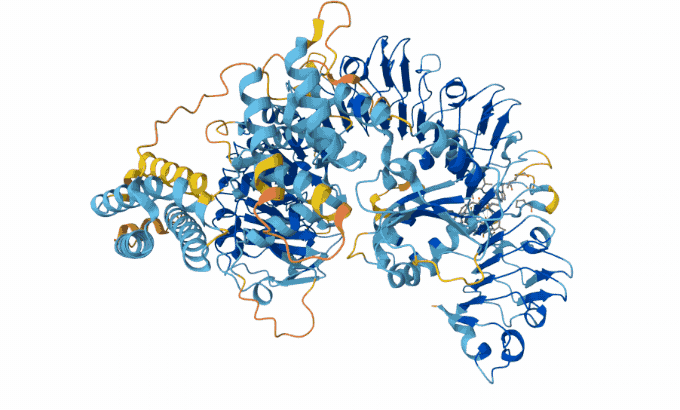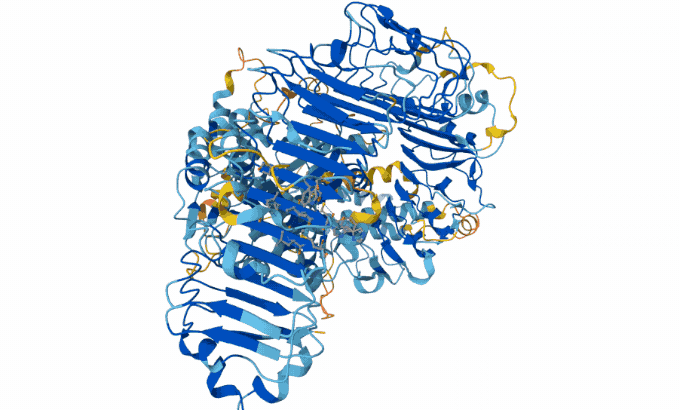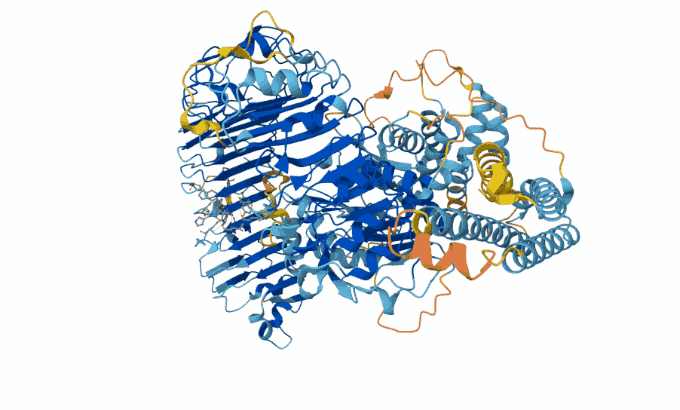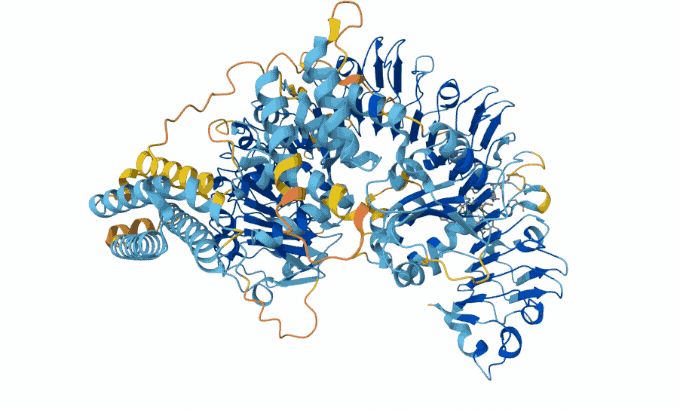
Hassabis and Jumper’s AI model taps into a database of known protein sequences and structures. It uses those data to map the likely physical distance between individual amino acids in a given protein string. Then it converts the map into a 3-D structure, Åqvist said — and with 90 percent accuracy. “Protein structure prediction with AlphaFold2 caused a complete revolution in structural biochemistry.”
The AlphaFold team has now predicted structures for almost all of the 200 million proteins that scientists know today. Biologists have used AlphaFold to predict proteins in sea cucumber genomes. It also predicted proteins that protect honeybees from bacterial infections.
Another Nobel for AI work
This is the second Nobel Prize this year that recognizes AI. The physics prize, awarded one day earlier, went to John Hopfield and Geoffrey Hinton. Their work helped pioneer the development of computer networks that were patterned on how neurons coordinate their processing in the human brain.
“It’s really interesting how well this aligns with the physics prize,” says Mary K. Carroll, president of the American Chemical Society. “The fact that neural networks were used by Hassabis and Jumper is another example of how computational and mathematical tools inform and underlie a lot of really exciting scientific work.”







