Virtual wounds: Computers probe healing
By giving virtual life to cells that ‘live’ in computers, scientists can probe how the body heals
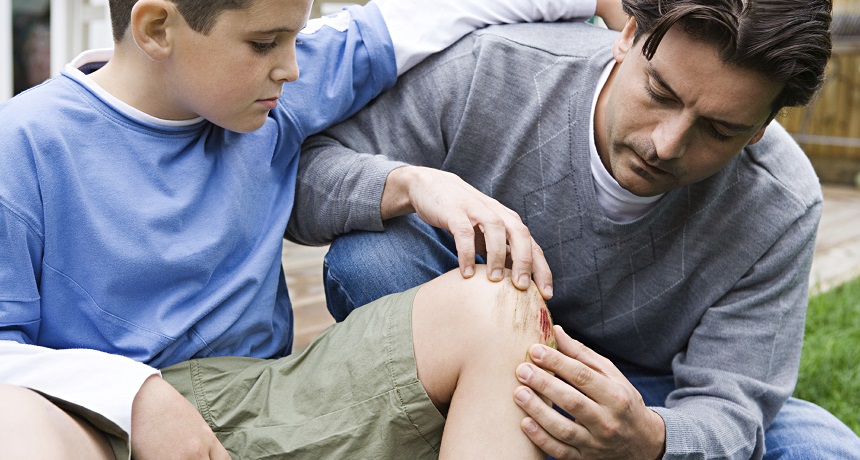
While this accident victim is focusing on the pain associated with his knee wound, his body is focusing on sending out immune troops — cells that can kill and discard invading germs.
IS¬_ImageSource/ iStockphoto
When you fall off your bicycle, slamming your knee into the pavement, your attention focuses on the pain. But the rest of your body is focusing on something else. Your injury has just begun rallying a large number of your cells to do serious battle.
Under your bruises and scratches, an army of fighter cells begins mobilizing. In the next few minutes to days, these troops will blast apart the body’s damaged cells and kill any invading bacteria. Then a follow-up cadre of different troops — think of them as the clean-up crew — arrives to tidy up the broken bits and pieces. Finally, medics move in to rebuild and heal the damaged tissue.
Scientists refer to these activities on the body’s battlefields as inflammation. The process is managed by the immune system. They include one of the body’s most complex families of cells.
Those inflammatory cells are efficient. They have to be. Their mission is to protect your health by destroying sick or damaged cells — as well as any intruders — before any of them can take over and spread disease.
But in their rush to save the body, these immune troops may make a few mistakes.
“Let’s say that you are a country and you are attacked,” says Gary An. He’s a surgeon and researcher at the University of Chicago, in Illinois. “You would kind of like to only kill the people who are your enemy,” he says. So your immune system drops what An calls “bombs” next to the enemy cells. These bombs are chemicals that break apart the problem cells.
But sometimes the bombs accidentally damage healthy cells, too. “You try to minimize that damage as much as you can,” he says. “But the damage needs to be strong enough to kill the invaders.” All too often, he says, this means “You end up destroying parts of your own country. That’s kind of what inflammation is.”
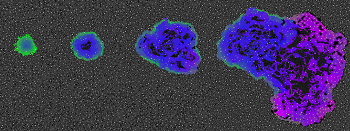
So scientists like An are trying to find better ways to understand the immune system. To do that, they’re borrowing a method pioneered in video games and the movies. It’s called agent-based modeling. Scientists study the process by essentially playing a computer game that attempts to mimic — or “model” — how the body works. They do this by creating virtual cells or “agents” that can interact inside the computer program.
Like a movie
It works like this. Imagine you’re helping make a movie. The director wants to shoot a battle scene with thousands of soldiers. Using real actors would be too expensive. So you propose using virtual soldiers. These can be created on a computer using special-effects software. Still, it would take forever to individually program each virtual soldier to participate in the scripted battle.
This challenge had to be solved for the movie The Lord of the Rings. To help create realistic battles using a large cast of virtual actors, the movie’s creators hired a computer company called WETA Digital. The company set up an agent-based model on a computer. The agents included virtual orcs, dwarves, humans and elves. The programmers gave each type of agent a set of rules.
Rules might tell an agent when to lift its sword, step backward, team up with a friend — or even do nothing. If an orc met a dwarf, for instance, the rule might tell the orc to swing its sword. When an orc instead met an elf, the rules might here instruct it to call in a friend to help in the fight. Each agent received hundreds of rules.
Yet when the designers hit “Go,” they got a surprise. The agents in the program didn’t just start fighting. One group of fighters banded together and fled the battleground. It was as if they were too scared to go on. No single rule told them to do this. The virtual fighters just combined several rules to make up their own new behavior.

Like An, Peirce uses agent-based modeling to study the immune system. Instead of orcs and elves, her agents are different types of immune cells. She assigns them rules and then lets them loose in a computerized “environment” that mimics the body. Amazingly, the agents indeed imitate how cells behave in the body, despite “living” in a computer. Scientists refer to this whole operation as a computer model.
“The cool thing about agent-based modeling is that it can allow us to have a ‘playground’ or a ‘sandbox’ where we can go in and make changes at the press of a button,” Peirce explains.
The war in your cells
Scientists usually study different parts (or systems) of the body in isolation — one part at a time. Then they probe it through a series of laboratory tests. With luck, this might show how it works. For instance, suppose you want to know if a drug will help cure brain cancer. You might put cancer cells and your drug into a test tube. If the drug kills the cancer cells, you might try the drug on normal brain cells in another test tube. If that drug doesn’t harm the normal cells, the drug might point to a cure.
But as in The Lord of the Rings, unexpected things can happen inside the body. For example, the drug might kill cancer cells, but also accidentally block normal brain cells from signaling other cells. Here, the brain might look healthy, but be unable to do its job.
In the immune system, each cell depends on all the other cells. Like players on a baseball team, the cells cooperate in ways that can’t be predicted by looking at just one player.
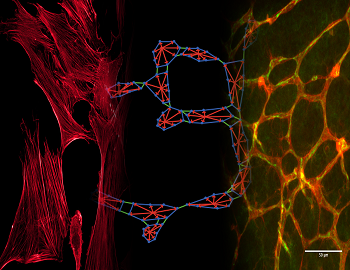
The first immune cells arriving to do battle are neutrophils (NU-troh-fils). These are a type of white blood cell. The bloodstream ferries them directly to the wound. The neutrophils lob chemical bombs at the bacteria, killing them and any damaged body cells.
These chemical bombs are not, however, very precise. They kill their targets — and neighboring healthy cells. So now, “You have to get someone to come in and clean it up,” says Joseph Walpole. He’s a graduate student in biomedical engineering who is studying with Peirce at the University of Virginia. If the damage is not cleaned up, those neutrophils will keep throwing bombs. In fact, any time a neutrophil sees damage, it throws another bomb.
Enter the janitors of the immune system: macrophages (MAK-row-FAY-zhehs). Like amoebas, these cells engulf and eat debris. Once the macrophages have gobbled up damaged cells and germs, the neutrophils view their job as over. As soon as the neutrophils toss their last bomb and the macrophages finish cleaning up, both cell types leave the battleground. Now, the area can begin to heal. Newly forming blood vessels deliver healer cells, such as platelets and fibroblasts. These cells build new tissue and heal the area.
Modeling those interactions — and maybe chaos
A researcher might activate bacteria, neutrophils and macrophages in an agent-based model. The environment surrounding the three agents is a patch of virtual human tissue. Rules might tell the virtual neutrophil how fast to arrive, how big a chemical bomb to make and when to lob it. Rules for the germ might include how long it takes to die once a bomb hits it. The computer allows scientists to change these inputs and watch what happens each time they tweak one of the rules.
If the neutrophils don’t put out a big enough bomb, harmful bacteria will take over. If the macrophages don’t arrive fast enough, the neutrophils will do too much damage and destroy the tissue they were meant to protect. And like the runaway fighters in The Lord of the Rings, the virtual cells sometimes do unexpected things in the agent-based model. These behaviors help show scientists surprising new possibilities for how cells might actually behave deep inside us.
As in this computer model, runaway inflammation can occur in real life. For some serious injuries, there’s so much damage that cells like macrophages can’t keep up. The neutrophils get overenthusiastic: They go bomb-crazy and take out too many healthy cells. This kills the patient in a process called sepsis. Here, it’s not the wound but the patient’s own immune response that proves fatal.
|
This video shows an agent-based model of an immune cell receiving chemical signals from other cells. The cell surface (blue), or membrane, contains proteins (red) that receive signals (white). Receiver proteins bind to the signals and then pass them to large proteins called enzymes embedded in the cell membrane (light blue and green squares). The enzymes bring the signals into the cell to act as messages (green cones) that instruct the cell to do different tasks. Gary An, Univ. of Chicago |
“Neutrophils are pretty dumb,” says An. “They don’t have the ability to know enough is enough.”
In the real immune system, many other types of cells join the neutrophils and macrophages. Each sends the others signals and instructions. If things go wrong, panic can occur.
Scientists can make more complex models to imitate this destructive chaos. An uses such a model to study sepsis. Some agent-based models have even been able to imitate the formation of a cancerous tumor.
On the horizon
Once a virtual tumor exists, scientists can probe how the immune system responds to it. Researchers at the Moffit Cancer Center in Tampa, Fla., have done just that. Alexander Anderson and his co-workers added interactions between immune-system cells to their virtual tumor. Their computer program showed that whether a tumor grew or shrank depended on how quickly two types of macrophages showed up.
One type cooperates with cancer cells. The other tends to destroy them. This still-unpublished discovery suggests that creating drugs to boost the activity of the destructive macrophages might offer a new way to treat some cancers.
Of course, agent-based modeling can’t solve all of medicine’s questions. Scientists still need to work in the laboratory with real cells. The more scientists learn about how cells work in a Petri dish or test tube, the more realistically they can set rules for virtual tissues. In the same way, agent-based models can suggest new things to try in the lab.
Vic DiRita studies microbiology and immunology at the University of Michigan in Ann Arbor. Speaking of agent-based modeling, he says: “I don’t know that it will or should replace experimental biology. But I think it’s a very powerful approach.” In fact, he says, “It’s impressive to be able to follow so many different agents … when each one of them is acted on by so many different forces.”
Using such models, the University of Chicago’s An has uncovered important features about sepsis. Damaged cells send out a special chemical signal. It’s like a distress cry. This signal attracts neutrophils. Some scientists thought they could stop sepsis in its tracks by giving patients a drug to block the signal. And in laboratory experiments, the drug indeed stopped neutrophils from coming.
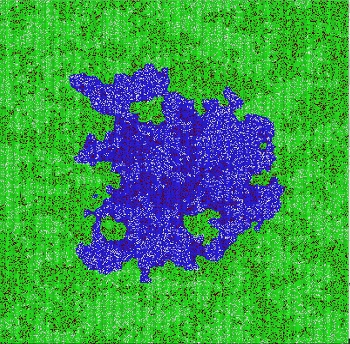
But using agent-based modeling, An also discovered why the drug would not save a septic patient. “What you get at the end of the time you give the drug is a bunch of damaged tissue,” says An. And, he adds: “It’s not like it will fix itself.”
Once the drug wore off, neutrophils returned. Inflammation started anew. “That’s the kind of secondary effect that’s incredibly obvious once you use the model,” observes An.
When it comes to the immune system, “it’s not just a sum of the parts,” says Virginia’s Walpole. “It’s not enough to have all the pieces. Agent-based modeling provides the possibility of looking at the interaction between the parts.”
Power Words
activate (in biology) To turn on, as with a gene or chemical reaction.
agent-based modeling The use of a computer to simlulate the many (potentially thousands of) players — or agents — in some process or event, each of which may have different rules about how they it function when confronted by particular circumstances.
amoeba A single-celled microbe that catches food and moves about by extending fingerlike projections of a colorless material called protoplasm. Amoebas are either free-living in damp environments or they are parasites.
bacterium (plural bacteria) A single-celled organism. These dwell nearly everywhere on Earth, from the bottom of the sea to inside animals.
cancer Any of more than 100 different diseases, each characterized by the rapid, uncontrolled growth of abnormal cells. The development and growth of cancers, also known as malignancies, can lead to tumors, pain and death.
debris Scattered fragments, typically of trash or of something that has been destroyed. Space debris includes the wreckage of defunct satellites and spacecraft.
digital (in computer science and engineering) An adjective indicating that something has been developed numerically on a computer or on some other electronic device, based on a binary system (where all numbers are displayed using a series of only zeros and ones).
environment The sum of all of the things that exist around some organism or process and the conditions they create for that organism or process. Environment may refer to the weather and ecosystem in which some animal lives, or, perhaps, the temperature, humidity and placement of components in some electronics system or product.
fibroblast A type of cell found in connective tissue; it makes and releases proteins important in wound healing.
germ Any one-celled microorganism, such as a bacterium, fungal species or virus particle. Some germs cause disease. Others can promote the health of higher-order organisms, including birds and mammals. The health effects of most germs, however, remain unknown.
immune system The collection of cells and their responses that help the body fight off infection.
immunity The ability of an organism to resist a particular infection or poison by producing and releasing special protective cells.
inflammation The body’s response to cellular injury and obesity; it often involves swelling, redness, heat and pain. It is also an underlying feature responsible for the development and aggravation of many diseases, especially heart disease and diabetes.
macrophage A type of white blood cell dispatched by the immune system. Like janitors of the body, they gobble up germs, wastes and debris for disposal. These cells also stimulate other immune cells by exposing them to small bits of the invaders.
model (in science) A simulation of a real-world event (usually using a computer) that has been developed to predict one or more likely outcomes.
neutrophil A type of white blood cell released by the immune system. It gobbles up wastes and release chemicals that can digest cells, including germs.
orc A fictional and villainous goblin-like humanoid found in the Lord of the Rings stories, which were created by J.R.R. Tolkien.
Petri dish A shallow, circular dish used to grow bacteria or other microorganisms.
platelets The smallest of blood cells, their role is to search for signs that a vessel has been damaged. Then they congregate at the site of damage and transform themselves, growing long tentacles. There they link together, creating a clot to plug any hole — stemming the potential loss of blood.
sepsis Complications of an infection that can prove life-threatening. It occurs when an inflammatory reaction gets out of control and risks affecting the entire body. Immune chemicals released into the bloodstream start damaging tissues throughout the body. Tissues affected by this runaway inflammation are called septic.
software The mathematical instructions that direct a computer’s hardware, including its processor, to perform certain operations.
tissue Any of the distinct types of material, comprised of cells, which make up animals, plants or fungi. Cells within a tissue work as a unit to perform a particular function in living organisms. Different organs of the human body, for instance, often are made from many different types of tissues. And brain tissue will be very different from bone or heart tissue.
tumor A mass of cells characterized by atypical and often uncontrolled growth. Benign tumors will not spread; they just grow and cause problems if they press against or tighten around healthy tissue. Malignant tumors will ultimately shed cells that can seed the body with new tumors. Malignant tumors are also known as cancers.
virtual Being almost like something. An object or concept that is virtually real would be almost true or real — but not quite. The term often is used to refer to something that has been modeled — by or accomplished by — a computer using numbers, not by using real-world parts. So a virtual motor would be one that could be seen on a computer screen and tested by computer programming (but it wouldn’t be a three-dimensional device made from metal).
white blood cells Blood cells that help the body fight off infection.
Word Find (click here to enlarge for printing)








