The newest elements finally have names
These superheavies honor the places or people instrumental in the discovery of such rare elements
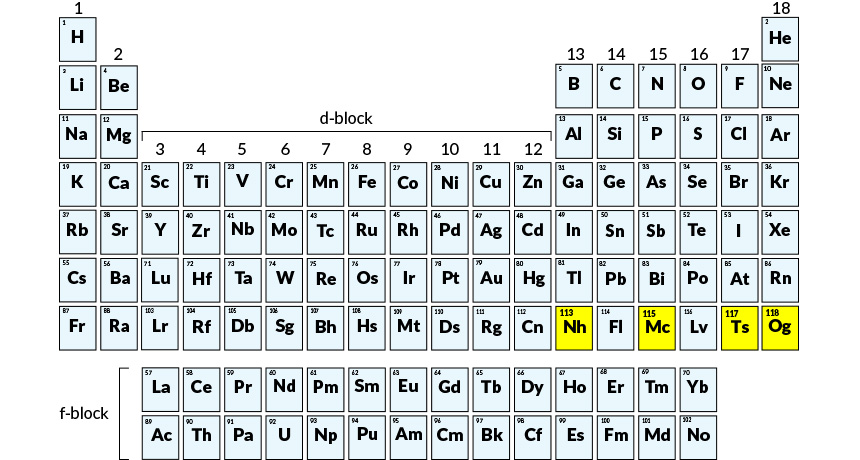
Four elements, officially added to the periodic table in December (and highlighted here in yellow), now have names that honor Japan, Moscow, Tennessee and a Russian physicist.
E. Otwell







