News Brief: Brrrrr — that’s really cold!
Physicists set new record low temperature
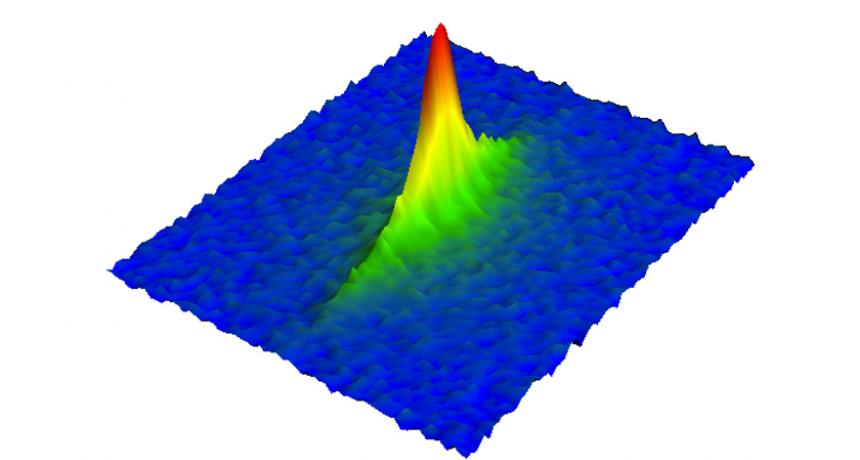
Stanford physicists used images like this one, showing a clumping of rubidium atoms, to determine that those atoms had achieved a record-low temperature.
T. Kovachy et al/Physical Review Letters 2015
By Andrew Grant
For a brief time, a swarm of atoms in Palo Alto, Calif., become the coldest stuff on Earth. Physicists in a Stanford University lab, there, brought them down to a super-frigid 50 trillionths of a kelvin. How cold is that? Well, on the kelvin scale, zero equals “absolute zero.” That’s simply as cold as anything can get. And in the new tests, the atoms almost got that cold. For comparison, the former record low was about 10 times as high, yet still far, far below even 1 kelvin.
The temperature of a sample depends on how fast its constituents move relative to each other. At absolute zero, for instance, they don’t move. At all. They’ll just sit in suspended animation. So to assess temperatures at the low end of the spectrum, scientists essentially measure atomic movement.
As a quantum physicist, Mark Kasevich studies the behavior of effects on the level of atoms and their building blocks. For the new study, his team worked with a cold gas. It consisted of about 100,000 rubidium atoms packed tightly in a cloud. Within a few seconds, the cloud began to spread apart. This was because some atoms in it were moving faster than others. At this point, the physicists zapped the rubidium cloud with a laser. This countered the atoms’ motion. The farther an atom had roamed (and thus the faster it had been moving), the more of a slowing nudge the laser gave it.
Eventually, all of them slowed to a crawl. The researchers described their achievement in the April 10 Physical Review Letters.
Ultracold atoms should lead to increasingly sensitive devices that can measure gravity and test the limits of quantum theory — an explanation for energy and matter as both operate at the level of individual atoms. Kasevich hopes to improve the super-chilling technique and cool atomic gases even more, to mere quadrillionths of a kelvin.
Power Words
(for more about Power Words, click here)
absolute zero The coldest possible temperature, also known as 0 kelvin. It is equal to minus 273.15 degrees Celsius (minus 459.67 degrees Fahrenheit).
atom The basic unit of a chemical element. Atoms are made up of a dense nucleus that contains positively charged protons and neutrally charged neutrons. The nucleus is orbited by a cloud of negatively charged electrons.
kelvin A temperature scale that has units the size of those on the Celsius scale. The difference, 0 kelvin is absolute zero. By contrast, 0 kelvin is equal to -273.15 Celsius. So 0 Celsius is equal to 273.15 kelvins. NOTE: Unlike with the Celsius and Fahrenheit scales, there is no use of the term “degrees” for numbers on the kelvin scale.
quantum theory A way to describe the operation of matter and energy at the level of atoms. It is based on an interpretation that at this scale, energy and matter can be thought to behave as both particles and waves. The idea is that on this very tiny scale, matter and energy are made up of what scientists refer to as quanta — miniscule amounts of electromagnetic energy.
quantum physics A branch of physics that uses quantum theory to explain or predict how a physical system will operate on the scale of atoms or sub-atomic particles.
rubidium Number 37 on the periodic table, German chemists discovered this element in 1861. Its presence created a deep red spectral line that showed up on an instrument (called a spectroscope). It had been used to analyze a mineral that contained this element. Rubidium’s name reflects the spectral surprise: It’s Latin for the deepest red.