Molecular scissors fix disease-causing flaw in human embryos
Scientists learned to use this gene-editing tool better to repair a gene that causes heart failure
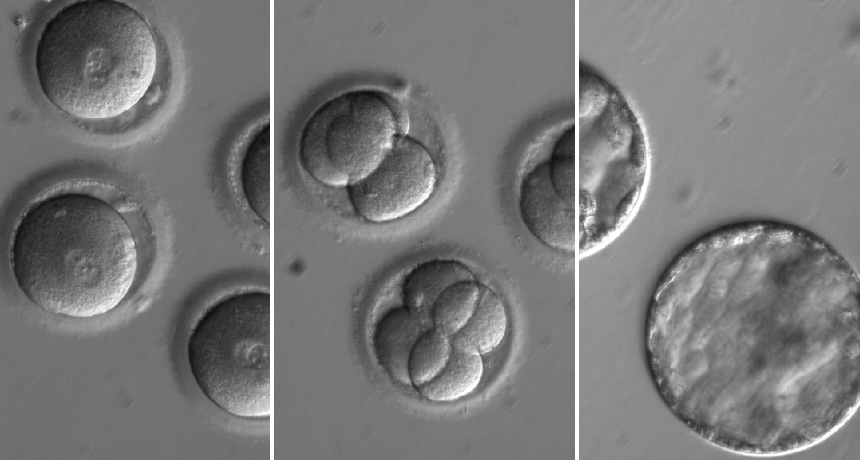
Researchers edited a gene that causes heart failure in newly fertilized eggs (left). Four-celled (center) and five-day-old embryos (right) look normal.
OHSU
Researchers used “molecular scissors” to mend a faulty gene in human embryos. These tests are the first of their kind in the United States. Scientists used an enzyme known as CRISPR/Cas9 as their scissors. It snipped out the faulty gene, which can lead to heart failure. With this tool, they fixed the problem in nearly three out of every four treated embryos (about 72 percent).
Such tinkering with an embryo’s DNA is known as germline editing. And it is controversial. Many people worry that it could be used to create so-called designer babies. Parents of these still-fictional designer babies might try to choose their child’s eye and hair color. Or they might have doctors change certain genes to make their child into a super-smart athlete.
In fact, the new study suggests such designer babies might be very hard to make. They would require injecting bits of new DNA to supply the desired traits. And in the latest tests, treated embryos rejected pieces of DNA that the researchers had added (in this case, to help fix the faulty gene).
The new work “is not announcing the dawn of the designer baby era,” says R. Alta Charo. She is a lawyer at the University of Wisconsin Law School in Madison. She also is a bioethicist, someone who studies the wisdom, morality or social value of certain decisions or policies. The researchers have not attempted to add any new genes or change traits, she notes. Here they sought only to repair one version of a disease-causing gene.
This research offers lessons in how to more accurately wield the CRISPR/Cas9 scissors. They reduced the share of embryos that were only partly edited.
That’s important if researchers want to use these gene scissors to cure genetic diseases, says Shoukhrat Mitalipov. He is a co-author of the new study. A reproductive biologist, he works at Oregon Health & Science University in Portland. If even one cell in an early embryo is unedited, he explains, “that’s going to screw up the whole process.” His team did its work in Korea, China and two U.S. states (Oregon and California).
They described their new achievements on August 2 in Nature.
Snip . . . then repair!
Mutations are changes that occur in a gene. Some mutations lead to disease. Others seem to cause no harm. In the new work, researchers wanted to fix a mutation in the gene known as MYBPC3.
Nearly every cell of the body has two copies of each gene. People who inherit just one mutant copy of MYBPC3 can develop a life-threatening heart problem. It’s called hypertrophic cardiomyopathy (HY-per-TROW-fik KAR-dee-oh-my-AH-puh-thee). It can cause sudden death from heart failure. The heart problem affects about one in every 500 people worldwide. Mutations in the MYBPC3 gene are to blame for about 40 percent of cases. Doctors can treat symptoms of the condition, but there is no cure.
In the new study, researchers used sperm from a man who carries a flaw in the MYBPC3 gene. They injected that sperm into eggs from women with healthy copies of the gene.
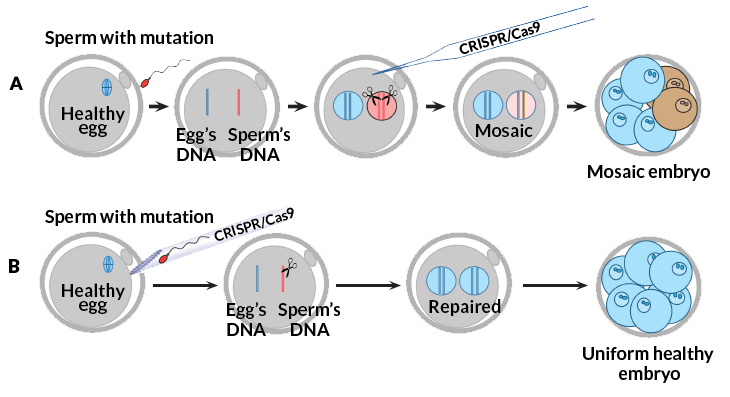
The researchers also injected the gene editor into the egg. The editor has several pieces. One is the scissors, a DNA-cutting enzyme called Cas9. They also need a piece of RNA that directs the enzyme to the exact place in the gene where a cut should be made. The researchers injected another piece of DNA into the egg, too. The researchers had designed that hunk of DNA to serve as directions for repairing the breach that would be made by Cas9. But the cells ignored it. Embryos instead used the mother’s healthy copy of the gene to mend the cut.
That result surprised the researchers. Gene editing in other types of cells usually requires an added piece of DNA as a pattern to guide a repair, Mitalipov says. If all embryos reject added DNA repair patterns, researchers may struggle to fix mutations in embryos. It would be hardest when neither parent has a healthy copy of the gene. The finding also means that embryos may reject attempts to add new traits. That might mean designer babies would be hard to achieve.
Story continues below image.
Careful timing
Timing is important in these experiments. At first, the researchers added the scissors one day after fertilizing the eggs. Those experiments often produced patchwork embryos — ones having some unedited cells. Thirteen out of 54 treated embryos had some unrepaired cells. Affected eggs that were fertilized had probably copied the DNA before researchers had added Cas9, Mitalipov now suspects.
When researchers injected the cutting enzyme and sperm at the same time, this didn’t occur. And none of the resulting embryos showed any signs that Cas9 had snipped a cut in the wrong place. (One concern about gene editing is that “off-target” cutting could create errors in other parts of a cell’s DNA.) When an egg was treated at fertilization, this way, only one patchwork embryo occurred. It had repaired the MYBPC3 mutation in all of its cells. Some cells had used the mother’s DNA for repair. Other cells used the DNA template supplied by the researchers.
The new study is a step toward using gene editing to prevent genetic diseases, says Janet Rossant. She is a developmental biologist in Canada at the Hospital for Sick Children and the University of Toronto. Still, she warns, researchers have a lot more work to do before they can do gene editing in people. Doctors will need to be sure the technique will work well and every time, she says.
Researchers have edited human embryos before. But they were merely probing the earliest stages of human development. They also wanted to learn things about basic human biology. Mitalipov and his colleagues instead wanted to probe the use of gene editing in medicine. They want to improve the technique so that it would be safe and reliable enough to use in people. Such studies might one day involve editing embryos in the lab, then implanting these into a women to establish a pregnancy.
In the United States, such human trials are not allowed. The Food and Drug Administration will not allow research meant to introduce changes in embryos that could be passed down to someone’s future children and grandchildren.







