Squeeze power
Under pressure, plastics produce energy
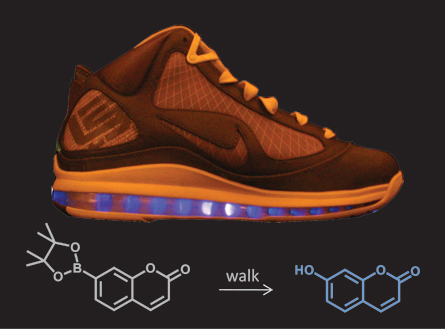
The “lightning shoe,” looks like an ordinary Nike LeBron sneaker. But it hides a secret that isn’t revealed until the sneaks have been for a 30-minute walk. Then, a liquid solution inside the sole begins to glow an eerie blue.
The engineers at Northwestern University in Evanston, Ill., who designed the new glow technology aren’t in the shoe business. Nor is their lightning model slated to hit stores in the near future. Rather, the researchers used this project as an example of a way to get energy from an unexpected source.
Plastic is made from a linked chain of identical molecules, and each molecule is a group of atoms. When the plastic bends or is squeezed, bonds that hold the atoms in place can break apart.
The bonds that hold the molecule together use pairs of negatively-charged particles called electrons. These particles always come in pairs. But once a bond breaks, an atom or molecule may be left with an odd number: It may lose an electron — or be left with an extra. Chemists call this fragment a free radical. It’s highly reactive, which means it wants to get back to an even number of electrons. It may steal an electron from a nearby atom, donate an extra electron or wedge itself into another molecule.

Air pockets in the shoe’s sole were injected with two ingredients: water and a chemical that glows when its molecules are broken apart. When the plastic sole is squeezed, such as when you step on it, it sets off a reaction. First, the plastic releases free radicals. The free radicals react with the water to form a substance called hydrogen peroxide.
Hydrogen peroxide is good at making reactions happen — that’s why it fizzes when you put it on a scraped knee or cut. The energy from the hydrogen peroxide can make other reactions happen, too — including making tiny amounts of a color-change chemical in the water glow.
The scientists report that energy also may be captured from squeezing a plastic sponge when it’s in a bucket of water. Or energy could be released from plastic bags made from this plastic as they’re squashed during recycling.
If tiny machines ran on power from sources like this one, they wouldn’t need batteries.
Speaking of such squash-energy devices, “people predicted that the energy efficiency would be minute,” Bartosz Grzybowski told Science News. Grzybowski, a physical chemist at Northwestern University, and his colleagues reported that their new method of collecting energy from plastic can be as efficient as coal-burning power plants are.
That’s good news for people interested in new energy sources, but free radicals aren’t always good news.
Set loose in the body, free radicals can damage cells and lead to health problems. That means that when implanted plastic devices (like catheters and breast implants) stretch or bend, they may release free radicals into the body. The experiment also suggests that the release of free radicals may help explain why some plastics break down with wear and tear.
POWER WORDS (adapted from the New Oxford American Dictionary)
free radical A charged molecule (typically highly reactive and short-lived) having one or more unpaired outer electrons.
atom The basic unit of a chemical element.
chemical bonds Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Some of the attractive forces are weak, some are very strong. All bonds appear to link atoms through a sharing of — or an attempt to share — electrons.
molecule A group of atoms held together by bonds.
electron A subatomic particle with a negative charge. Electrons act as the carriers of electricity.
physical chemistry The area of chemistry that uses the techniques and theories of physics to study chemical systems.







