Explainer: CO2 and other greenhouse gases
Carbon dioxide is just one of several chemicals that contribute to the greenhouse effect

As this offshore natural-gas platform burns off one greenhouse gas — methane — it produces another, carbon dioxide.
Theerapong28/iStockphoto
Many different gases make up Earth’s atmosphere. Nitrogen alone accounts for 78 percent. Oxygen, in second place, makes up another 21 percent. Many other gases comprise the remaining 1 percent. Several (such as helium and krypton) are chemically inert. That means they don’t react with others. Other bit players have the ability to act like a blanket for the planet. These have come to be known as greenhouse gases.
Like the windows in a greenhouse, these gases trap energy from the sun as heat. Without their role in this greenhouse effect, Earth would be quite frosty. Global temperatures would average around -18° Celsius (0° Fahrenheit), according to the National Oceanic and Atmospheric Administration (NOAA). Instead, the surface of our planet averages around 15 °C (59 °F), making it a comfy place for life.
Since about 1850, though, human activities have been releasing extra greenhouse gases into the air. This has slowly propelled a rise in average temperatures across the globe. Overall, the 2017 global average was 0.9 degree C (1.6 degrees F) higher than it had been between 1951 and 1980. That’s based on calculations by NASA.
Stephen Montzka is a research chemist with NOAA in Boulder, Colo. There are four main greenhouse gases to worry about, he says. The best known is carbon dioxide (CO2). The others are methane, nitrous oxide and a group that contains chlorofluorocarbons (CFCs) and their replacements. (CFCs are refrigerants that have played a role in thinning the planet’s protective high-altitude ozone layer. They are being phased out as part of a global agreement begun in 1989.)
Many chemicals influence climate. However, Montzka notes, these four greenhouse gases are the ones “that we [humans] have direct control over.”
Climate-warming chemicals
Each greenhouse gas, once emitted, rises into the air. There, it helps the atmosphere hold onto heat. Some of these gases trap more heat, per molecule, than do others. Some also stay in the atmosphere longer than others. This is because each has different chemical properties, Montzka notes. They also are removed from the atmosphere, over time, by different processes.
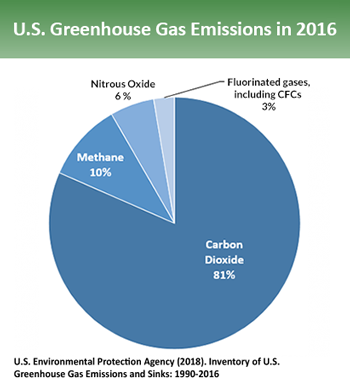
Excess CO2 comes mainly from burning fossil fuels — coal, oil and natural gas. Those fuels are used for everything from powering vehicles and generating electricity to manufacturing industrial chemicals. In 2016, CO2 accounted for 81 percent of the greenhouse gases emitted in the United States. Other chemicals are more effective at trapping heat in the atmosphere. But CO2 is the most abundant of the ones released by human activities. It also sticks around longest.
Some CO2 gets removed each year by plants as they grow. However, much CO2 is released during colder months, when plants aren’t growing. CO2 also can be pulled from the air and into the ocean. Organisms in the sea can then convert it into calcium carbonate. Eventually that chemical will become an ingredient of limestone rock, where its carbon can be stored for millennia. That rock-forming process is really slow. Overall, CO2 can linger in the atmosphere for anywhere from decades to thousands of years. So, Montzka explains, “even if we stopped emitting carbon dioxide today, we would still see warming from that for a very long time.”
Methane is the main component of natural gas. It’s also released from a host of biological sources. These include rice production, animal manure, cow digestion and the breakdown of wastes put into landfills. Methane accounts for about 10 percent of U.S. greenhouse-gas emissions. Each molecule of this gas is much better at trapping heat than is one of CO2. But methane does not remain in the atmosphere as long. It gets broken down as it reacts in the atmosphere with hydroxyl radicals (neutrally charged OH ions made from bound atoms of oxygen and hydrogen). “The timescale for methane removal is about a decade,” Montzka notes.
Nitrous-oxide (N2O) made up 6 percent of greenhouse gases emitted by the United States in 2016. This gas comes from agriculture, the burning of fossil fuels and human sewage. But don’t let its small quantity make you disregard N2O’s impact. This gas is hundreds of times more effective than is CO2 at trapping heat. N2O also can linger in the atmosphere for nearly a century. Each year, only about 1 percent of airborne N2O gets converted by green plants into ammonia or other nitrogen compounds that plants can use. So this natural N2O removal “is really slow,” Montzka says.
CFCs and their more recent replacements are all manufactured by people. Many have been used as refrigerants. Others are used as solvents for chemical reactions and in aerosol sprays. Together, these made up only about 3 percent of U.S. greenhouse gas emissions in 2016. These gases are only removed when they get locked up in a high layer of the atmosphere. In this stratosphere, high-energy light bombards the chemicals, breaking them apart. But that can take decades, Montzka says.
Fluorine-based chemicals, such as CFCs, he notes, “are potent greenhouse gases, on a per molecule basis.” But releases of them are so low that compared to CO2, their overall impact is quite small. Reducing emissions of methane, N2O and CFCs will help slow climate change, Montzka notes. “But if we’re going to solve this [greenhouse gas] problem, we need to take care of CO2,” he says. “It’s contributing the most … and it has this extremely long residence time in the atmosphere.”







