Amoebas are crafty, shape-shifting engineers
They can change form, build shells, punch holes in prey — even farm their own food
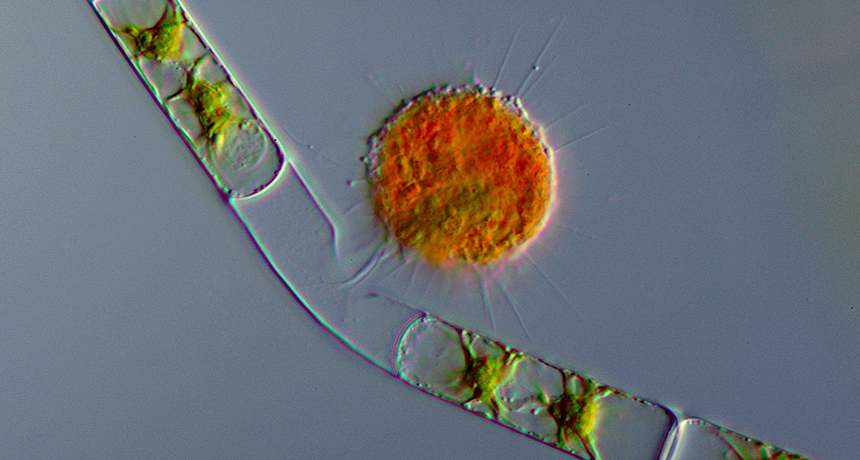
An amoeba (in orange) named Vampyrella lateritia finishes eating the contents of an algal cell (green). Though often overlooked, amoebas have an amazing range of unusual behaviors.
Sebastian Hess
By Roberta Kwok
In 2009, biologist Dan Lahr received an intriguing email from another researcher. It included a photo of a strange organism. The researcher had discovered the microbe in a floodplain in central Brazil. Its yellowish-brown shell had a distinctive, triangle-like shape.
The shape reminded Lahr of the wizard’s hat in The Lord of the Rings movies. “That’s Gandalf’s hat,” he remembers thinking.
Lahr is a biologist at the University of São Paulo in Brazil. He realized the one-celled life form was a new species of amoeba (Uh-MEE-buh). Some amoebas have a shell, as this one did. They may build those shells out of molecules they make themselves, such as proteins. Others may use bits of material from their environment, such as minerals and plants. Still other amoebas are “naked,” lacking any shell. To learn more about the newfound amoeba, Lahr would need more specimens.

Two years later, another Brazilian scientist sent him pictures of the same species from a river. But the bonanza came in 2015. That’s when a third scientist emailed him. This researcher, Jordana Féres, had collected a few hundred of the triangular amoebas. It was enough for her and Lahr to begin a detailed study of the species.
They examined the microbes under a microscope. The amoeba, they found, built its hat-shaped shell from proteins and sugars that it made. The big question is why the microbe needs that shell. Perhaps it offers protection from the sun’s harmful ultraviolet rays. Lahr named the species Arcella gandalfi (Ahr-SELL-uh Gan-DAHL-fee).
Lahr suspects many more amoeba species await discovery. “People are just not looking [for them],” he says.
Scientists still know little about amoebas. Most biologists study organisms that are either simpler or more complex. Microbiologists, for instance, often focus on bacteria and viruses. Those microbes have simpler structures and can cause disease. Zoologists prefer to study larger, more familiar animals, such as mammals and reptiles.
Amoebas have largely “been ignored,” notes Richard Payne. He is an environmental scientist at the University of York in England. “They’ve been sort of caught in the middle for a long time.”
But when scientists do peer at these odd little organisms, they find big surprises. Amoebas’ foods range from algae to brains. Some amoebas carry bacteria that protect them from harm. Others “farm” the bacteria they like to eat. And still others may play a role in Earth’s changing climate.
What’s on the menu? Fungi, worms, brains
Though you can’t see them, amoebas are everywhere. They live in soil, ponds, lakes, forests and rivers. If you scoop up a handful of dirt in the woods, it will probably contain hundreds of thousands of amoebas.
But those amoebas may not all be closely related to one another. The word “amoeba” describes a wide variety of single-celled organisms that look and behave a certain way. Some organisms are amoebas for only part of their lives. They can switch back and forth between an amoeba form and some other form.
Like bacteria, amoebas have just one cell. But there the similarity ends. For one thing, amoebas are eukaryotic (Yoo-kair-ee-AH-tik). That means their DNA is packed inside a structure called a nucleus (NEW-klee-uhs). Bacteria have no nucleus. In some ways, amoebas are more similar to human cells than to bacteria.
Also unlike bacteria, which hold their shape, shell-free amoebas look like blobs. Their structure changes a lot, Lahr says. He calls them “shape-shifters.”
Their blobbiness can come in handy. Amoebas move by using bulging parts called pseudopodia (Soo-doh-POH-dee-uh). The term means “false feet.” These are extensions of the cell’s membrane. An amoeba can reach out and grab some surface with a pseudopod, using it to crawl forward.
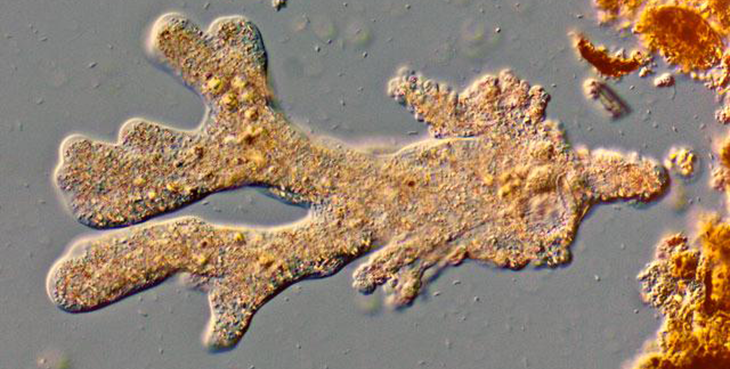
Pseudopodia also help amoebas eat. A stretched-out pseudopod can engulf an amoeba’s prey. That allows this microbe to swallow bacteria, fungal cells, algae — even small worms.
Some amoebas eat human cells, causing sickness. In general, amoebas don’t cause as many human diseases as bacteria and viruses do. Still, some species can be lethal. For example, a species known as Entamoeba histolytica (Ehn-tuh-MEE-buh Hiss-toh-LIH-tih-kuh) can infect human intestines. Once there, “they literally eat you,” Lahr says. The disease they cause kills tens of thousands of people each year, mostly in areas that lack clean water or sewer systems.
The most bizarre illness caused by an amoeba involves the species Naegleria fowleri (Nay-GLEER-ee-uh FOW-luh-ree). Its nickname is the “brain-eating amoeba.” Very rarely, it infects people who swim in lakes or rivers. But if it gets inside the nose, it can travel to the brain where it feasts on brain cells. This infection is usually deadly. The good news: Scientists know of only 34 U.S. residents who became infected between 2008 and 2017.
A tiny can opener
A scientist named Sebastian Hess recently discovered the tricks some amoebas use to eat. He studies eukaryotic microbes in Canada at Dalhousie University. That’s in Halifax, Nova Scotia. Hess has loved watching tiny critters through a microscope since he was a kid.
Ten years ago, Hess punched through the ice of a frozen pond in Germany. He collected a sample of water and took it back to his lab. Through the microscope, he saw something odd. Green spheres were wiggling like tiny bubbles inside strands of green algae. He had “no idea” what the spheres were. So Hess mixed algae containing the green balls with other algae. The wiggling spheres popped out of the algae and started swimming. Shortly afterward, they invaded other algal strands.
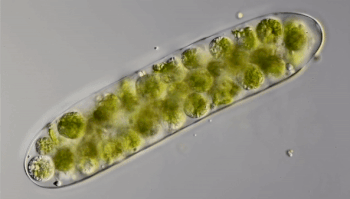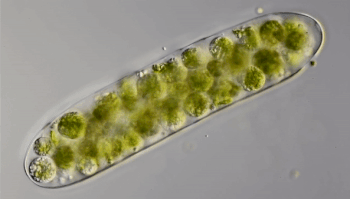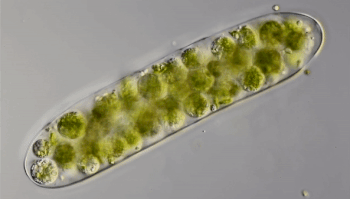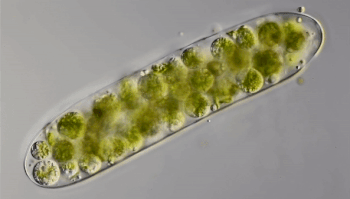
Hess realized that the green spheres were microbes called amoeboflagellates (Uh-MEE-buh-FLAH-juh-laytz). That means they can switch between two forms. In one form, they swim or glide using tail-like structures called flagella (Fluh-JEH-luh). When the swimmers find food, they transform into amoebas. Their shape becomes less rigid. Instead of swimming, they now begin crawling along some surface.
Through the microscope, Hess watched one of these amoebas cut a hole in an algal cell. The amoeba squeezed inside. Then it ate the alga’s innards. Afterward, the amoeba divided and made copies of itself. Those were the wiggling green spheres that Hess had seen earlier. The new amoebas punched more holes in the algal cell. Some invaded the neighboring cell in the algal strand. Others escaped. Hess named the species Viridiraptor invadens (Vih-RIH-dih-rap-ter in-VAY-denz).
He found a similar species in a bog. Also an amoeboflagellate, it didn’t crawl inside algae. Instead, it cut a C-shaped gash in an algal cell. Hess likens this amoeba to “a can opener.” The amoeba then lifted the “lid” and used its pseudopod to reach into the hole. It gobbled up the material it pulled out of the cell. Hess named this species Orciraptor agilis (OR-sih-rap-ter Uh-JIH-liss).
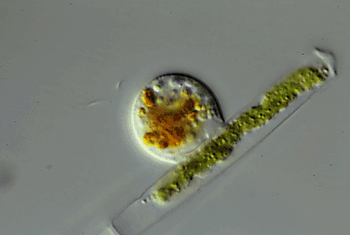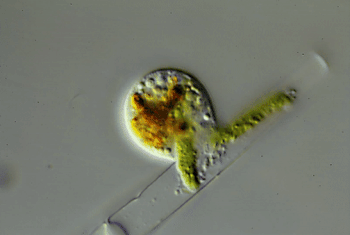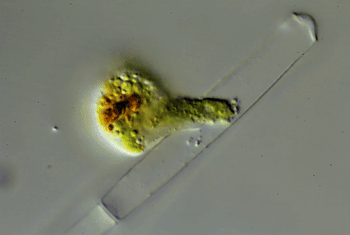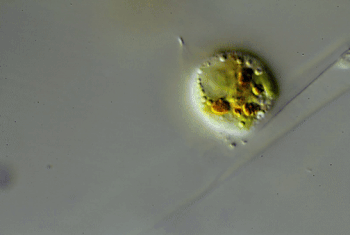
More recently, he discovered clues to how these two amoeboflagellates hack into algae. Both seem to get help from a protein called actin (AK-tin). Human cells use the same protein to move.
In amoeboflagellates, actin forms a mesh. It helps the cell make a pseudopod. The mesh might also help the pseudopod latch onto algae. Actin can connect to other proteins in the microbe’s cell membrane that might attach to the walls of algal cells. Actin may even help guide other proteins — enzymes — that can cut into algal cell walls.
Results from studies by Hess and his colleagues suggest that these seemingly simple amoebas may be far more advanced than they first seemed. One might even consider them one-celled engineers. “In terms of their behavior,” Hess says, “they are just super-complex organisms.”
Bacterial buddies
The relationship between amoebas and bacteria is even more complicated.
Debra Brock is a biologist at Washington University in St. Louis, Mo. She studies an amoeba called Dictyostelium discoideum (Dihk-tee-oh-STEE-lee-um Diss-COY-dee-um). Many simply refer to them as Dicty. These soil-dwelling organisms dine on bacteria.
Dicty usually live solo. But when food is scarce, tens of thousands may merge, clumping into a dome. Usually, the dome morphs into a slug-like shape. This slug — really thousands of individual amoebas moving together — crawls toward the soil surface.

Once it gets there, the slug forms a mushroom shape. Amoebas at the top of the “mushroom” surround themselves with a hard coat. This coated form is known as a spore. Insects, worms or larger animals that brush against these spores may unknowingly transport them to new places. Later, the spores will crack open, allowing the amoebas inside the coat to strike out in search of food at this new site.
Some Dicty bring bacteria along for food. They carry the bacteria inside themselves without digesting them. It’s “like a lunch box,” Brock explains. To do this, the amoebas get help from a different group of bacteria that they can’t eat. These helper microbes also live in the amoebas. The helpers prevent the food bacteria from being digested so that the amoebas can save them for later.

Scientists call the bacteria-carrying amoebas “farmers.” Researchers suspect that when the amoebas reach a new home, they spit the food bacteria out into the soil. Those bacteria then divide to make more bacteria. It’s like the amoebas are carrying seeds and planting them to grow more food.
Recently, researchers discovered that the amoeba slug protects itself with special cells while it’s traveling. These cells are also Dicty amoebas. Known as sentinel cells, they mop up bacteria and toxic substances that could harm the other amoebas. When that’s done, the slug leaves its sentinels behind.
Brock wondered what that finding meant for Dicty farmers. The farmers wouldn’t want sentinel cells to kill their bacterial food. So did farmers have fewer sentinel cells than non-farmers?
To find out, Brock’s team let amoeba slugs form in the lab. Some slugs were all farmers. Others were all non-farmers. Researchers dyed the sentinel cells, then let the slugs move across a lab dish. Afterward, the researchers counted how many sentinel cells had been left behind. As expected, farmer slugs had fewer sentinel cells.
The scientists wondered if this put farmers at greater risk from toxic chemicals. To test that, Brock exposed farmers and non-farmers to a toxic chemical. The farmers could still reproduce. In fact, they fared better than non-farmers.
Brock now thinks that some of the bacteria carried by the farmers helped to fight off the toxic chemicals. These bacteria might break down the chemicals. So farmers have two weapons against toxic threats: sentinel cells and bacterial buddies.
A link to climate change?

Hess and Brock study naked amoebas. Payne is intrigued by those with shells. Called testate (TESS-tayt) amoebas, these crafty microbes can fashion many types of shells. Those coverings can resemble discs, bowls — even vases. Some are “fantastically beautiful,” Payne says.
Many testate amoebas live in habitats called peat bogs. These sites are usually soggy and acidic. But during summers, the peat can dry out. Payne thinks shells might protect a bog’s amoebas during these droughts.
Not just curiosities, these peat-dwelling amoebas may play an important role in the environment, Payne says. Partly decayed plants build up in peat bogs. Bacteria eat those plants, releasing carbon-dioxide gas. In the atmosphere, that greenhouse gas can foster global warming. Bog amoebas eat these bacteria. So in that way, a bog’s amoebas may influence how big a role peatlands play in global warming.
Payne and his colleagues studied one peat bog in China where a wildfire had burned. Wildfires may become more frequent as the climate warms. So the scientists wanted to know how fire affected the bog’s testate amoebas.
Payne’s Chinese colleagues took samples from burned and unburned parts of the bog. Then the team analyzed differences between two types of testate amoebas. One makes its shell out of debris, such as sand grains and bits of plants. The other type builds a glassy shell using a mineral called silica.
In unburned patches, the scientists found similar numbers of both types of amoebas. But burned patches contained many more amoebas with shells made of sand and debris. The findings suggest that the fire had destroyed more of the amoebas with silica shells.
Payne doesn’t yet know what that means for climate change. It’s not clear whether the shift in amoebas will cause peat bogs to release more or less carbon. The process is “hugely complicated,” he says.
Many other details about amoebas remain unknown. How many species exist? Why do some have shells? How do amoebas affect the numbers of other microbes in some parts of the environment? How do they influence the ecosystem around them, such as plants?
Scientists have enough questions about amoebas to occupy themselves for a long time. That’s partly why researchers such as Payne find these organisms so intriguing. Plus, he says, “They’re just really cool.”








