Superslim silicene
Lab-made sheets of silicon are only one atom thick
You’re never far from silicon. This material is used to make the computer chips inside cell phones, computers, MP3 players and other electronic devices. Even if you don’t use these devices, silicon is just beneath your feet: It makes up more than one-quarter of the weight of the Earth’s crust.
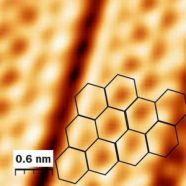
Silicon comes in many forms, from large rock crystals to tiny grains of sand. Now there’s one more: Scientists have created sheets of silicon that are only one atom thick. This creation is called silicene. If you zoom in far enough with a powerful microscope, each sheet looks like a honeycomb. Both structures are made of connected hexagons.
At a scientific meeting in Dallas on March 24, physicist Antoine Fleurence presented a new way to make silicene. Fleurence and his colleagues at the Japan Advanced Institute of Science and Technology in Ishikawa found a way to create silicene slices. When they used X-rays to study their creation, they saw the familiar honeycomb pattern.
Though silicon is in the ground, don’t go looking for silicene sheets there: They’re so thin they are invisible to the naked eye. And the sheets can be forged only in the laboratory — not in nature. The physicists who made silicene were inspired by graphene, a similar material that was first found in 2004. Like silicene, graphene has the thickness of a single atom. Graphene also has a honeycomb shape. Unlike silicene, which is made of silicon atoms, graphene is made of carbon atoms.

At the time of its discovery, graphene was the thinnest and most expensive material in the world. Since then, scientists have found new and cheaper ways to make the material. Graphene is strong and flexible, and it conducts electricity. Scientists envision using this wonderstuff for portable electronics — like electronic paper you can roll up and tuck into your pocket.
Graphene is considered so important that in 2010, Andre Geim and Konstantin Novoselov, the physicists from the University of Manchester who first isolated the material, received the Nobel Prize in Physics. Geim and Novoselov, as well as other teams of physicists, have gone on to build new and small electronic components from graphene.
But silicene may give graphene a run for its money. In a 2007 study, researchers from Wright State University in Dayton, Ohio, showed that if silicene were created, it could be expected to have some of the same properties as graphene. (These researchers came up with the term silicene.) Plus, Fleurence says, manufacturers already know how to work with silicon.
“Silicon has the advantage of being more [useful] in today’s electronics,” he said at the March meeting in Dallas.
Silicene has a few more hurdles to overcome before it can compete with graphene. Scientists need to continue testing the material’s ability to conduct electricity. And silicene also needs to be easy to make. The scientists who first made graphene used only Scotch tape and a graphite material similar to pencil lead.
There’s no similarly easy experiment behind silicene, but that doesn’t mean it’s not worth watching. In the future, silicene could be a big player in the world of small electronics.
POWER WORDS (adapted from the New Oxford American Dictionary and researchers at Wright State University in Dayton, Ohio, who coined the term silicene)
silicon A material that can sometimes conduct electricity. Silicon is widely used to make electronic circuits.
semiconductor A material that sometimes conducts electricity. Semiconductors are important parts of computer chips.
silicene A superthin material made from a single layer of silicon atoms connected together.
graphene A superthin, superstrong material made from a single layer of carbon atoms connected together.
carbon A nonmetal element that has two main forms (diamond and graphite) and that also occurs in impure form in charcoal, soot and coal.
atom The basic unit of a chemical element.







