Form some bonds with a chemistry card game
A new game challenges players to pair positively and negatively charged elements for points
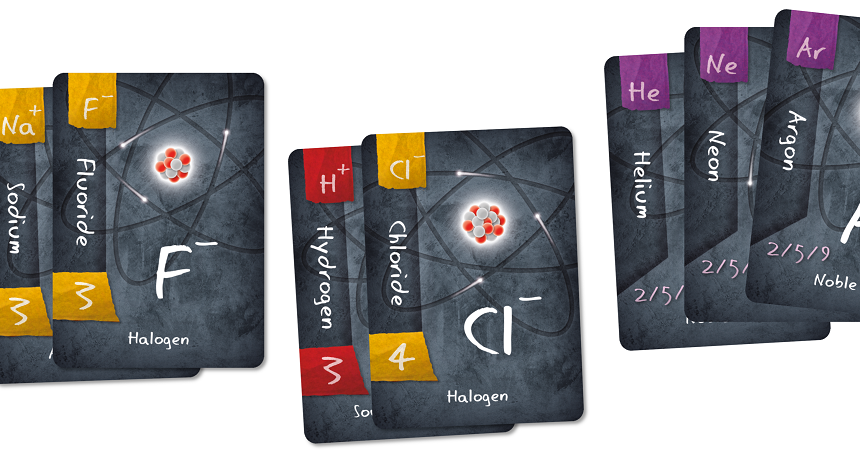
This card game lets you form some bonds between chemicals. Along the way, you might even find yourself bonding with your friends.
John Coveyou
Learning some concepts in chemistry can require a lot of studying. It can be hard to memorize chemical reactions and keep track of atoms — the basic units of chemical elements — and their building blocks. A great way to learn some of this is to make the work a game — as in Ion. It’s a new card game.
Ions are atoms or molecules that have electric charge due to losing or gaining electrons. Those electrons are negatively charged subatomic particles that orbit an atom’s nucleus. The game challenges players to pair positively and negatively charged ions to create compounds, which are substances made from two or more chemical elements. Players who earn the most points making compounds will win the game.
The game starts as a player deals out eight cards to each person. Then, each player selects a card from the batch he or she was dealt. After putting it on the table, this person passes the rest of their cards to the player on the left. In the next stage, the players do the same, working from their seven remaining cards.
GOOD CHEMISTRY This video describes the basic rules for playing Ion, a game which challenges players to form chemical bonds to earn points. John Coveyou |
From now on, players need to select a card that will pair with one or more that he or she had selected earlier. A clever player will also select cards that will leave opponents’ ions high and dry. The goal is to collect groups of cards that will create neutral compounds — ones with no charge. Each successful chemical compound earns players points for the round. Players also can collect the stand-alone noble gases for points. Three “action tiles” per person allow players to take more than one card, to steal cards from their neighbors or to acquire cards from a central pool.
You don’t have to be a chemistry whiz to enjoy the game. The cards show the charges associated with each ion. Players can simply add and subtract the charges to make sure they are forming bonds correctly. Separate compound cards offer descriptions of the different chemicals that players can make and what they are used for — from the sodium chloride in your salt shaker to the sodium fluoride in your toothpaste.
Designed by John Coveyou of Genius Games, Ion has just been funded on Kickstarter. It can be preordered online here until May 6, 2015.
Follow Eureka! Lab on Twitter
Power Words
(for more about Power Words, click here)
atom The basic unit of a chemical element. Atoms are made up of a dense nucleus that contains positively charged protons and neutrally charged neutrons. The nucleus is orbited by a cloud of negatively charged electrons.
chemistry The field of science that deals with the composition, structure and properties of substances and how they interact with one another. Chemists use this knowledge to study unfamiliar substances, to reproduce large quantities of useful substances or to design and create new and useful substances. (about compounds) The term is used to refer to the recipe of a compound, the way it’s produced or some of its properties.
chlorine A chemical element with the scientific symbol Cl. It is sometimes used to clean water. Compounds that contain chlorine are called chlorides.
compound (often used as a synonym for chemical) A compound is a substance formed from two or more chemical elements united in fixed proportions. For example, water is a compound made of two hydrogen atoms bonded to one oxygen atom. Its chemical symbol is H2O.
electron A negatively charged particle, usually found orbiting the outer regions of an atom; also, the carrier of electricity within solids.
element (in chemistry) Each of more than one hundred substances for which the smallest unit of each is a single atom. Examples include hydrogen, oxygen, carbon, lithium and uranium.
fluoride Achemical, such as sodium fluoride, that contains the element fluorine. In small doses, fluorides can help prevent tooth decay.
ion An atom or molecule with an electric charge due to the loss or gain of one or more electrons.
sodium A soft, silvery metallic element that will interact explosively when added to water. It is also a basic building block of table salt (a molecule of which consists of one atom of sodium and one atom of chlorine: NaCl).







