Lego-like way to snap molecules together wins 2022 chemistry Nobel
Three chemists pioneered this 'click chemistry' for the lab and for use in living cells
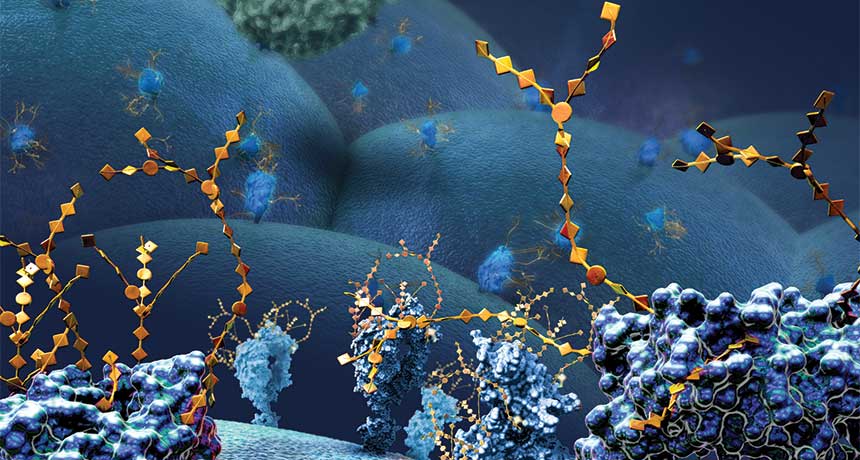
To track cells, scientists can add Lego-like chemicals into sugars on the surface of those cells. Here, large surface proteins with chains of sugars (illustrated, gold) are shown on the outside of a cancer cell. It’s a form of the “click” technology that won the 2022 Nobel Prize in chemistry.
NICOLLE RAGER FULLER
By Meghan Rosen and Nikk Ogasa
Imagine a tool kit for snapping together molecules like Lego building blocks. That’s essentially the development that brought three chemists this year’s Nobel Prize in chemistry.
The Royal Swedish Academy of Sciences in Stockholm announced the award at a news conference on October 5.
With her win, Carolyn Bertozzi of Stanford University in California becomes only the eighth woman to take home a Nobel in this field. She shares it with Morten Meldal at the University of Copenhagen in Denmark and Barry Sharpless of the Scripps Research Institute in La Jolla, Calif. The trio won for pioneering what’s being described as click chemistry. Meldal’s and Sharpless’ “click tool” allows scientists to easily build complex molecules in the lab. Bertozzi’s work allowed it to also be used inside living organisms.
“The good thing with this discovery is that it can be used for almost everything,” says Olof Ramström. He’s a chemist at the University of Massachusetts Lowell and a member of the Nobel committee for chemistry. The new techniques can be used to build drug molecules, polymers and other new materials. They also can be used to track selected molecules in the body.
“I think this chemistry is going to revolutionize medicine in so many areas,” says Angela Wilson of Michigan State University in East Lansing. (She’s also president of the American Chemical Society, in Washington D.C.) In terms of using these techniques, she notes, “We’re kind of at the tip of the iceberg.”
Around 20 years ago, Sharpless introduced the idea of click chemistry. It’s a way to simply and quickly attach two compounds using certain connector molecules. But finding Lego-like connectors to attach those building blocks wasn’t easy. On their own, Sharpless and Meldal each discovered the solution. They added a smidge of copper to a mixture containing two other small molecules. This let the scientists rapidly snap the two small molecules together into a ring-shaped chemical.
This reaction quickly “gained enormous interest,” Ramström notes. Even though scientists would later discover a handful of other molecules that could snap together in the same fashion, that first reaction is considered the “crown jewel of click reactions.”
While triggering those reactions with copper may work fine in a glass beaker, that metal can harm living cells. Bertozzi discovered a way to do copper-free click chemistry. Now scientists can use it to make certain chemical reactions take place in living things — without mucking up their cells’ normal activities.
Making click chemistry safe for cells
Bertozzi’s specialty has been studying sugar molecules. These “are incredibly difficult to work with,” explains Leslie Vosshall. She’s a neuroscientist at Rockefeller University in New York City. She also is the vice president and chief scientific officer at the Howard Hughes Medical Institute. Direct methods exist for looking at DNA, RNA and proteins. The same has not been true for sugars, she notes. “Sugars are the dark matter of the cell.”
Bertozzi’s Nobel-winning innovation was to trick cells into incorporating a click chemical into the sugars that adorn their outer surface. Now, when scientists expose these cells to a different click chemical, the two will snap together. It’s just like the molecules do in Sharpless’ and Meldal’s reactions. By linking cells to green-glowing molecules, for instance, scientists can now make the cells’ surfaces glow.
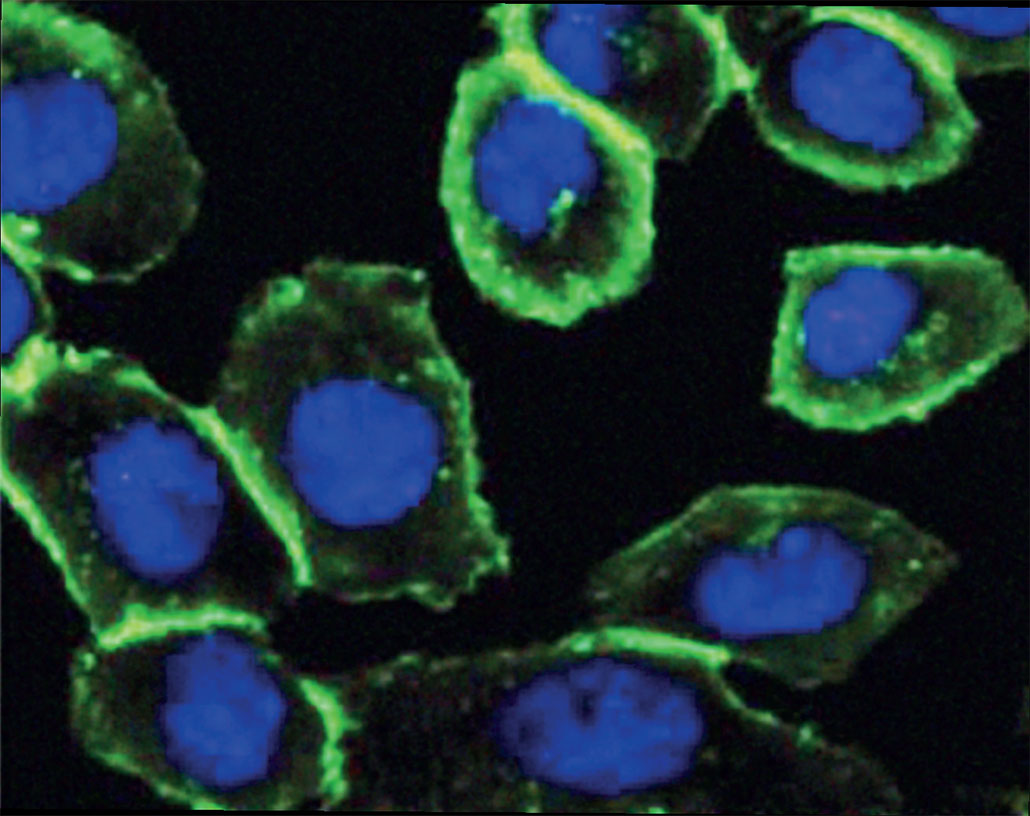
If you could attach shining molecules to things in a living cell “then you could follow them in a microscope and see where they are and how they move,” notes Johan Åqvist. “This is what Carolyn Bertozzi did,” the chemist explains. Åqvist works at Uppsala University in Sweden. He also chairs the Nobel committee for chemistry.
By targeting specific sugars on cell surfaces, scientists can develop new treatments. For instance, Bertozzi and her colleagues were able to target — and turn off — sugars that can help tumor cells hide from the immune system’s T cells.
“Carolyn is … one of the astonishingly few women in chemical biology,” says Vosshall. Her lab “has inspired women chemists and put them out into the world.”
Bertozzi woke up to a 3 a.m. phone call alerting her she was a Nobel winner. On hearing the news, she recalls being “absolutely stunned. I’m sitting here and can hardly breathe.” Saying this middle-of-the-night phone call was a shock is an understatement, she added. “I’m still not entirely positive that it’s real. But it’s getting realer by the minute.”
Bertozzi, Meldal and Sharpless will share 10 million Swedish kronor. (That’s worth roughly $900,000.) The win is the second for Sharpless. He also shared the 2001 Nobel prize in chemistry. That win was for his work on a new type of catalyst.







