Blue light flexes its chem-building muscle
Engineers used light to combine two polymers into one stronger, more flexible material
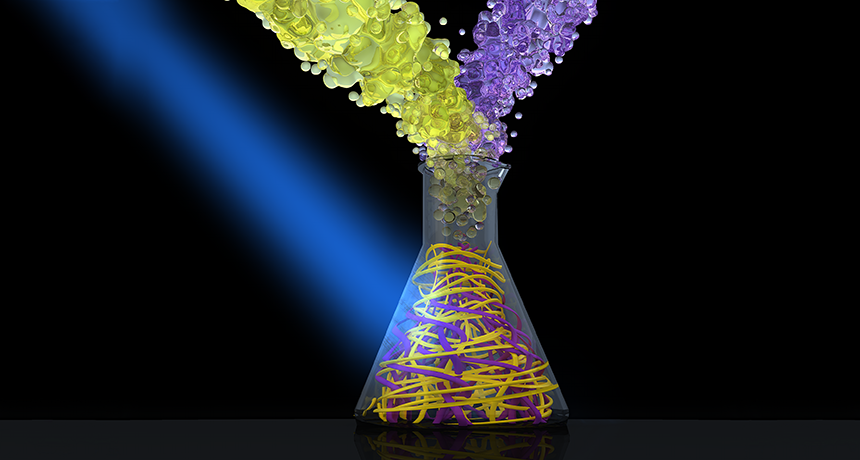
Engineers used blue light to trigger the formation of two polymers in the same mixture. This created a new material that’s both strong and flexible.
A.U. Shete and C.J. Kloxin/Polymer Chemistry 2017
By Sid Perkins
A new material can hold up to stress, bend a lot without breaking and remember its shape. Researchers created it by blending the ingredients for two polymers, then triggering their formation with a beam of blue light. The finding, researchers say, could help scientists develop all sorts of new materials. That includes everything from longer-lasting dental fillings to scratchproof coatings.
Polymers are long molecules made of repeating series of smaller building blocks. The term polymer comes from the Greek words for “many parts.” Think of a polymer as a chain. Each of the chain’s links is called a monomer. In Greek, that means “one part.” In some cases, polymers look like branching networks rather than single chains.
Different polymers have features that make them useful for different purposes. For example, some polymers are strong. They can resist being pulled, pushed or twisted. But strong polymers are often brittle. That means they can snap without warning, notes Christopher Kloxin. He’s a chemical engineer at the University of Delaware in Newark. For some uses, engineers would like a polymer that’s strong but not brittle, he says. So Kloxin and a graduate student, Abhishek Shete, tried to make one.
Previously, people have tried to make such a material by blending two polymers — a strong one and a flexible one. They would mix the ingredients for both in one container. Then they would try to coax them to lengthen and mix consistently into one new material. Unfortunately, that hasn’t always worked out.
Sometimes one polymer forms far more quickly than the other one. That allows a lot of the monomers for the slowly-forming one to leave the mix before they have a chance to bond, notes Kloxin.
His team’s solution? Find polymers that play nicely together.
After many attempts, Kloxin and Shete turned to blue light as a way to control the polymer-building. They chose two monomers that start to form chains when exposed to this light. One makes a brittle polymer called a polyacrylate (PAA-lee-AK-rih-layt). The other monomer produces more flexible chains.
The end result was a glass-like film that combines the best features of its components. Kloxin and Shete described their technique in the June 28 issue of Polymer Chemistry.
Blue-light blend
The researchers mixed the ingredients for the two polymers along with a small amount of two added chemicals. Known as initiators, those last two chemicals help to get the chemical reactions going. Then they beamed blue light onto the mix. The light triggered both polymers to start forming, but at slightly different rates.
The more flexible polymer began forming first. Within 30 minutes, about 85 percent of its monomers had formed polymer chains. After 10 minutes, more than 90 percent of the acrylate monomers had combined into polymers.
Each type of polymer strand also can make additional chemical bonds — known as crosslinks — with others that had been made from the same starting monomers. (Think of the polymers, once formed, as very tiny strands of blue and red spaghetti bond together in the same pot. Red strands bond to form red mats and blue strands bond with other blues.) All of the polymer chains tangle together, forming a network within the new material.
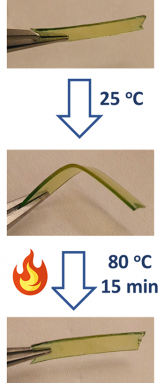
Besides being both strong and flexible, the new material has another cool feature. It features what’s known as shape “memory.” If someone bends it into a new form, something made from the new material will, if heated, slowly return to its original shape. In the lab, Kloxin and Shete bent a sample that was 0.5 millimeters (0.02 inch) thick into an L shape. Then they heated it to 80° Celsius (176° Fahrenheit). After 15 minutes, the sample was once again flat.
“This is really neat work,” says Christopher Bowman. He’s a chemical engineer at the University of Colorado Boulder. Researchers have tried to mix these types of materials before, he notes. But, he adds, those efforts haven’t worked as well.
Because the new process used light to form the mix of polymers, it typically can build only thin films this way. Explains Bowman, light triggers the reaction. So the polymers will start forming along the outer surface of the mixture, where the light will be most intense. As the polymers form, they will tend to block some of the light from reaching deeper into the mix. But there is a potential way around this, Bowman notes. The team could probably use the material to build up a tough but flexible object using 3-D printing, he says. This process creates three-dimensional objects in a series of thin layers. That often involves exposing a liquid to some particular wavelength of light that will make it harden.
The team’s new technique could help make better scratch-resistant coatings or longer-lasting dental fillings, Bowman suspects. “The possibilities are quite exciting.”







