Bones have stealth role in muscle, appetite and health
More than a scaffold for our tissues, bones help orchestrate the activities of many organs

The bones that support muscles and other tissues as we run, jump and reach out have some hidden properties too. They send out hormones that can affect appetite, disease risk and how our bodies use food.
Halfpoint/iStockphoto
Long cast as the strong silent type, bones aren’t as quiet as they once seemed. It turns out they have a lot to say. And the rest of the body appears to be listening.
Scientists knew that bones are not just rigid scaffolds to hold up and protect soft tissues. The bones themselves are ever-changing. Day in and out they’re destroying old cells and then creating new ones. This constant turnover, known as remodeling, keeps bones strong and healthy.
Hormones are naturally secreted chemicals. They act like long-range messengers within the body, instructing some tissue to take up some task — and at just the proper time. Several hormones, for instance, are key to directing the remodeling of bones. Emerging data now suggest that bones don’t just inform other bones about when to do something. Studies in mice show that bone hormones chat with a host of organs and tissues, including the brain, kidneys and pancreas.
“There’s so much going on between bone and brain and all the other organs,” says Beate Lanske. She is a bone and mineral researcher at the Harvard School of Dental Medicine in Boston, Mass. Bone was once considered a “dead organ,” she notes. No more. Their active release of chemical signals now suggest bones may actually resemble glands — the tissues that release hormones.
Clifford Rosen is an endocrinologist (En-doh-krin-OLL-oh-gizt) at Maine Medical Center in Scarborough. He studies how bone hormones affect health and disease. He says, “The skeleton must have some fine-tuning mechanism.” That, he adds, must be what “allows the whole body to be in sync with what’s happening at the skeletal level.”
Bones require a lot of energy as they constantly remodel themselves. Cells known as osteoblasts make new bone. Others, known as osteoclasts, destroy old bone. So it makes sense for bones “to have things to regulate the fuel sources that are necessary for bone formation,” Rosen notes. Osteoblasts and osteoclasts can use hormones for this.
He points to a recent demonstration in mice of a bone-brain link. Bones unleash the hormone lipocalin 2 (LCN2) to stem bacterial infections. But a study now shows that LCN2 also works in the brain to control hunger.
Story continues below image.
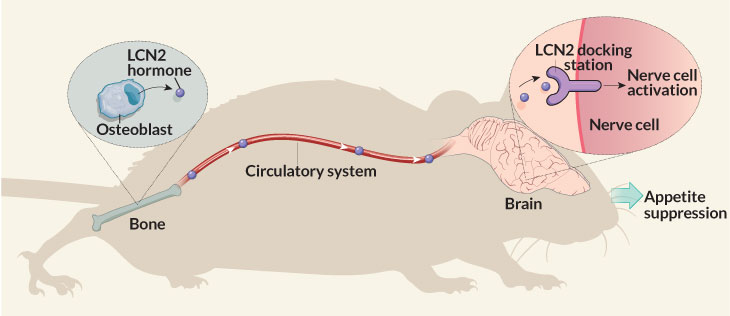
After a meal, a mouse’s bone-forming cells absorb nutrients and release LCN2 into the bloodstream. This hormone now travels to the brain. There it gloms onto hunger-controlling nerve cells. They tell the brain to stop eating, Stavroula Kousteni explains. A physiologist, she works at Columbia University Medical Center in New York City.
She and her colleagues reported the new finding March 16 in Nature.
Researchers had thought fat cells made most LCN2. But in mice, bones produce up to 10 times as much of this hormone as fat cells do. After a meal, their bones pumped out enough LCN2 to boost blood levels of the hormone to triple what they had been before the meal. Concludes Kousteni, appetite control is “a new role for bone.”
At least three other bone hormones also talk to organs other than bone. These hormones are osteocalcin, sclerostin and fibroblast growth factor 23. Scientists have only begun to eavesdrop on the conversations bones are having with such other tissues. And that snooping suggests bone chatter can play a role in controlling how the body manages sugar, energy and fat.
Wide-ranging effects
Scientists began homing in a decade ago on the chemicals that bones release as their messages. Gerard Karsenty is a geneticist at Columbia University Medical Center. He has discovered many links between the bone-hormone osteocalcin and metabolism, which is how the body uses food to fuel its activities. In 2007, he reported that osteocalcin helps keep blood-sugar levels in a healthy range.
Osteocalcin circulates in the blood. There, it collects calcium and other minerals that bones need. At least in mice, when the hormone reaches the pancreas, it tells cells to ramp up production of another hormone: insulin. This is the hormone that the body uses to move sugar out of the bloodstream and into cells, where it can fuel activities.
That same bone hormone that ups insulin production also tells fat cells it’s time to release a hormone that increases the body’s sensitivity to insulin. Diabetes is a serious disease where the body either makes too little insulin or comes to ignore the presence of much of its insulin. If osteocalcin works the same way in people as it does in mice, Karsenty says, it could become a potential drug to treat diabetes or obesity.
“Their data is fairly convincing,” says Sundeep Khosla. He is a bone biologist at the Mayo Clinic in Rochester, Minn. “But the data in humans has been less than conclusive,” he adds. Many things can affect how the body manages blood sugar, he notes. In people, it has been hard to show whether osteocalcin is a major player.
Mouse data also suggest that osteocalcin may play a role in how the body uses food, its energy source. After an injection of the hormone, old mice can run as far as younger mice. Old mice that didn’t receive an osteocalcin boost ran only about half as far, Karsenty and his colleagues reported last year in Cell Metabolism. As the hormone increases endurance, it helps muscles absorb more nutrients. Muscles respond by telling bones to make more osteocalcin.
Karsenty’s group has found hints that this same effect seems to take place in people. For instance, women’s blood levels of osteocalcin increase during exercise, they note.
Rodent data from the Karsenty lab suggest osteocalcin can do even more. This bone hormone stimulates cells in the male reproductive organs to pump out testosterone. That’s a hormone crucial for strength, bone density and reproduction. It may also improve mood and memory.
Bones might even use the hormone to talk to the brain of a fetus in the womb. Osteocalcin from the bones of pregnant mice can penetrate the womb to help shape the developing brain. That’s something that Karsenty’s team reported four years ago in Cell. What benefit bones get from influencing developing brains remains unclear.
Keertik Fulzele is a molecular biologist at Boston University in Massachusetts. His team has turned up another bone messenger that may affect metabolism: sclerostin (Sklair-OS-tun). This hormone’s day job is to tell bone-forming cells to slow down or stop. But bones may also dispatch this hormone to manage fat, an important energy source. In mice, sclerostin helps convert white (or “bad”) fat into more useful energy-burning beige fat. Fulzele’s team reported this in the February 2017 issue of the Journal of Bone and Mineral Research.
Protecting bones
The activity of yet one more bone hormone seems to show promise in understanding a perplexing health condition. That hormone: fibroblast (FY-bro-blast) growth factor 23.
Bones use this FGF-23 to help the kidneys regulate blood levels of phosphate. That’s a mineral that serves as a major building block of bones and teeth. If the body has more phosphate than the bones need, those bones release FGF-23. It informs the kidneys to trash the excess phosphate by excreting it in the urine. But in people with kidney failure, cancer or some genetic diseases, FGF-23 levels can soar when they shouldn’t. This mistakenly encourages the body to dump too much phosphate. Bones then weaken as they become starved of the mineral.
Suzanne Jan De Beur is an endocrinologist at Johns Hopkins Bayview Medical Center in Baltimore, Md. There, she is studying an inherited disease in which FGF-23 is a major player. Known as XLH, this genetic disease leads to soft bones. In XLH, a missing or broken gene in bones causes a flood of FGF-23. De Beur’s team is testing a way to soak up the extra hormone to keep a patient’s bones from becoming too soft.
A clinical trial is a way to test whether a new drug is safe and effective against a disease. In March, De Beur’s group completed the first part of such a trial in adults with XLH. As a Phase III trial, this type serves as one of the final tests to see if a drug is reliable and safe enough to get government approval for use in patients.
The scientists are working with the drug company Ultragenyx. That company’s drug latches onto extra FGF-23 before the hormone can reach the kidneys. The drug acts “like a decoy in the blood,” says Lanske, who is not involved in the trial. When FGF-23 runs into the drug, it mistakenly attaches to it instead of going to the kidneys. Once connected, the duo gets broken down by the body.
Traditionally, doctors have treated XLH patients by giving them extra phosphate, notes De Beur. But it’s like trying to fill a bathtub without a plug, she says. The kidneys are peeing it out about as fast as we can pour it in the mouth. No surprise: It doesn’t always work. Plus, long-term treatment can cause severe side effects.
The trial researchers hope the new drug will help restore the body’s ability to absorb phosphate. Unpublished data indicate it just may work. Of the 68 people who got the drug, at least 9 in every 10 had normal blood phosphate levels after six months of treatment. People taking the drug also reported less pain and stiffness than those not on the drug, Ultragenyx announced in April.
Story continues below table.
Odd Jobs
Bones produce hormones that affect the activity of other organs. Some roles for these hormones are well known. Other new roles have only recently begun to emerge.
| Hormone | Known Function | Proposed functions | Site of proposed activity |
|---|---|---|---|
| Lipocalin 2 (LCN2) | Stops the spread of bacterial invaders | Regulates appetite | Brain |
| Osteocalcin | Regulates bone renewal and calcium use | Regulates: Blood sugar and Memory and mood Testosterone |
Pancreas and fat tissue Brain Testicles |
| Sclerostin | Keeps bone growth in check | Plays role in food use by the body | Fat tissue |
Bone-brain connection
If results in animals apply to people, osteocalcin, sclerostin and LCN2 might one day also be used to treat diseases.
In a recent study, Kousteni’s team found that boosting LCN2 levels in mice that lacked the LCN2 gene tamed their voracious feeding habits. Even in mice with working LCN2 genes, injecting them with extra amounts of this hormone made them eat less. It also lowered their blood sugar and increased their sensitivity to insulin. These last two traits suggest this hormone might one day be used to treat people with type 2 diabetes.
Researchers traced LCN2’s path from the bones to the brain’s hypothalamus (HY-poh-THAL-uh-mus). This part of the brain helps maintain healthy blood-sugar levels and body temperature. Injecting LCN2 into mice’s brains made them less hungry appetite and cut their weight gain. The hormone attaches to the surface of nerve cells that regulate appetite, the team now proposes.
To work, the LCN2 must insert itself into a receptor, or docking station, on brain cells. So mice whose cells had defective LCN2 docking stations responded differently. They ate too much. And they gained weight just like mice that couldn’t make the hormone in the first place. Injections of LCN2 didn’t curb their eating or weight gain.
Some early work suggests LCN2 may play a similar role in people with type 2 diabetes.
In a small group of patients, those who were overweight had less LCN2 in their blood. And a few people whose brains had defective LCN2 docking stations had higher blood levels of the bone hormone. This suggests LCN2 had nowhere to attach in the brain. So it built up in the blood.
If the hormone suppresses appetite in people, it could be a great obesity drug, Rosen says. But it’s too early to make any firm conclusions about its potential role as a medicine for people.
Yet one thing is clear, he says. The era of calling bone a silent bystander is over.







