Boosting Fuel Cells
Young scientists tackle projects to improve energy sources for cars and homes.
By Emily Sohn
Some people are happy to learn simply for the sake of learning. Megan Burger would rather use her education to create cutting-edge inventions with important uses.
That’s exactly what the 18-year-old has done. At this year’s Intel International Science and Engineering Fair (ISEF) in Phoenix, Ariz., the high school senior from The Woodlands, Texas, was one of several students who investigated fuel-cell technology. Her work might eventually help make Earth a cleaner place.
 |
|
Fuel cells power this car, which runs on hydrogen. |
| U.S. Department of Energy |
Fuel cells generate electricity through chemical reactions between hydrogen and oxygen. The process is an efficient way to produce energy with no toxic waste.
Fuel cells already power spacecraft and a few cars. In the future, experts suggest, they will also be used in homes and other buildings and even in devices such as laptops and cell phones.
The technology has “the potential to transform the [U.S.] energy industry,” Megan says.
Still, there are problems. Even though there’s more hydrogen in the universe than any other element, it’s difficult to gather and store.
On Earth, most hydrogen is attached to oxygen (as in water molecules, which contain two atoms of hydrogen for every atom of oxygen) or to other atoms, and energy is required to separate them. That’s an issue if the point is to generate energy without the pollution produced by burning gasoline, oil, coal, and other fuels used today, which might be needed to free up hydrogen.
Stronger, safer
For her project, Megan wanted to create a fuel cell that would be stronger and safer than those now available.
First, she did a lot of background reading, using books and the Internet, just to understand the basics of such a complicated topic. “I had to do a lot of research,” she says.
 |
|
Megan Burger and her fuel cell project. |
| Emily Sohn |
One of the first things Megan learned is that a fuel cell has three main parts that fit together like a sandwich. The “bread” on one side is called an anode. The other slice of “bread” is called a cathode. The “meat” squished between the cathode and the anode is called an electrolyte. There are lots of different types of fuel cells, which differ mainly in the kinds of electrolytes they use.
In her experiments, Megan worked with a type of fuel cell called a solid-oxide fuel cell (SOFC), which has a solid electrolyte. SOFCs, which are stronger and safer than liquid versions, are used today in power plants, and engineers are looking for ways to use them in laptops and cell phones, Megan says. The downside is that they require high temperatures—around 1,000 degrees Celsius—to work. This limits where the fuel cells can be used.
“I thought maybe I could make them operate at lower temperatures,” Megan says.
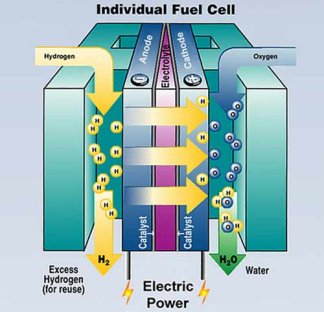 |
|
As hydrogen and oxygen flow through a fuel cell, they generate electricity and produce water. The fuel cell’s core is an electrical sandwich that consists of an anode, an electrolyte, and a cathode. |
| U.S. Department of Energy |
For 2 years, Megan experimented with various electrolyte materials to lower the temperature at which an SOFC operates. Eventually, she ended up with solid pellets of a material called bismuth strontium barium oxide. No one had ever used this material before in a fuel cell, she says. Her tests showed that a fuel cell based on that material would be able to operate at a mere 650 degrees Celsius.
“That’s already 350 degrees less than before,” Megan says. “It gives me hope that we can have another step down.”
Her research continues. In the meantime, she has already learned more than she ever expected to learn—and not just about fuel cells.
“One of the biggest lessons is perseverance,” she says. “If you want to find something exciting, you have to keep trying, no matter how many things around you are breaking, exploding, or not working. Thousands of things that people call failures are still things you can learn from.”
Cold start
ISEF participant Wade Miller learned some important lessons from his fuel cell project, too. And like Megan, Wade didn’t know where he was going to end up when he began.
“It was really confusing when I first started learning about it,” says Wade, a 17-year-old senior from Albert Lea, Minn. Still, the concept was exciting enough to keep him going.
 |
|
Wade Miller holds his fuel-cell-powered toy vehicle. |
| Emily Sohn |
Because Wade lives in Minnesota, a state with hot summers and cold winters, he wanted to test a fuel cell’s ability to withstand temperature extremes. His main goal was to figure out whether cars powered by fuel cells might some day be practical where he lives.
Unlike Megan, Wade used a polymer electrolyte membrane (PEM) fuel cell. These types of fuel cells work at relatively low temperatures, and they use sheet-like electrolytes that resemble plastic wrap.
To test efficiency at various temperatures, Wade built a toy car and attached a PEM fuel cell to it. That way, he could measure how much energy the cell produced. He tested the system under heat lamps at 80 degrees F, indoors at 65 degrees F, outdoors at 27 degrees F, and in the school’s freezer at –10 degrees F.
Wade had predicted that the fuel cell would work best at higher temperatures. Instead, it produced the most electricity at –10 degrees F.
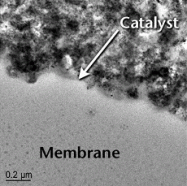 |
|
Polymer electrolyte membrane (PEM) fuel cells use sheets of plastic as electrolytes. The cathode and anode are typically made from carbon, with a layer of platinum, which serves as a catalyst to speed up the separation of electrons from hydrogen atoms. |
| U.S. Department of Energy |
“I ended up proving myself completely wrong,” he says. “If you start a car in Minnesota when it’s really cold, it takes a while for the engine to turn over. I thought the fuel cell would do the same. But I guess not.”
In the end, Wade concluded that fuel-cell-powered cars would run just fine in Minnesota. Still, he predicts, it’ll be another 10 or 15 years before we actually see them on the streets.
“This is probably something we will deal with in the future,” he says. “It’s a good thing to get into. We will be seeing this in the news.”
And today’s young scientists are already on the case. As usual, taking a walk around the exhibition hall at this year’s ISEF was like taking a walk into the future.
Going Deeper: