Building a better battery
Researchers develop a way to make batteries that hold more charge and don’t weaken with age
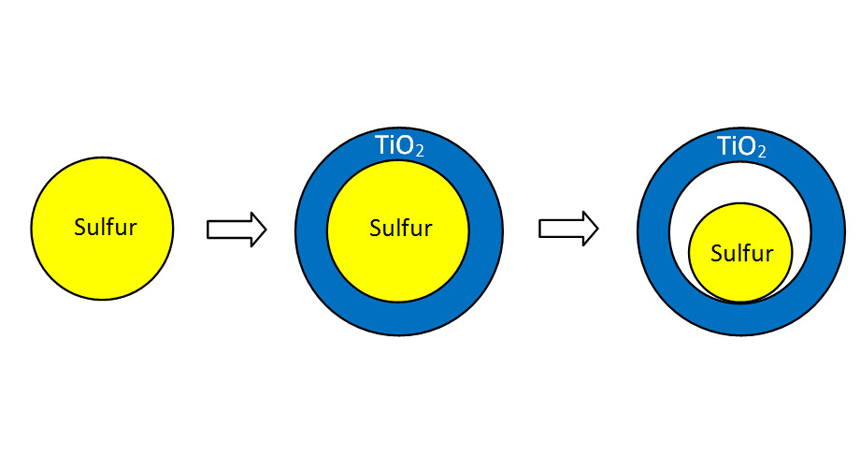
Scientists created small particles of sulfur (left) and then coated them with titanium dioxide (blue). Then they dissolved and washed away some of that sulfur. This made room in the particle for byproducts that form when a sulfur-based battery is charged. If not given space, these byproducts can crowd a battery and make it explode.
SEH ET AL., NATURE COMMUNICATIONS (2013)
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
By Sid Perkins
Battery designers face many challenges, but two stand out. One is developing small, heavy-duty batteries. These units hold a really big charge. The other is being able to recharge such batteries hundreds of times without them losing their ability to hold a boatload of energy. A team of scientists now reports having achieved both feats. It involved adding sulfur to their battery’s recipe.
Oh, and unlike earlier attempts to build sulfur-based batteries, these aren’t prone to explode.
The new design could someday lead to smaller, longer-lasting batteries, scientists say.
Batteries, especially those for powering portable electronics, need to store a lot of energy in a small space. This trait lets people use devices longer before needing to recharge the battery, explains Yi Cui of Stanford University in California. As a materials scientist, Cui studies how the atomic and molecular structure of a material relates to its overall properties. Cui worked on the team that designed the new sulfur-based battery.
Sulfur is a very attractive battery material, he notes. Just a small amount of this inexpensive yellow element can pack a lot of energy. But past attempts to use it in batteries have proven difficult. The reason: Each time you charge a sulfur-based battery, bad byproducts form.
What makes them bad? These byproducts — known as polysulfides — take up more room than the starting sulfur. In some cases, says Cui, they can take up almost double the volume of the original sulfur. And an increase that large could make a battery explode.
Another problem: These byproducts readily dissolve in the fluids found inside most batteries. That, in turn, makes it increasingly harder to recharge the battery back to full power.
No question, says Hector Abruña, “Using sulfur in batteries is a complex problem from many standpoints.” A chemist at Cornell University in Ithaca, N.Y., he did not work on the new project.
But Cui’s team has come up with a way to solve both the expansion of the byproducts and their mixing throughout the battery.
First, the scientists developed bits of sulfur that were each 800 nanometers across. Nano is a prefix meaning a billionth. So each of these particles was about 800 billionths of a meter wide. (They are so small that 20 sitting side by side wouldn’t be as wide as the diameter of the thinnest human hair!) The scientists then gave each bit a 15-nanometer-thick coating. That thin coat was made from titanium dioxide. This inexpensive material is used in a range of products, including paints and puddings.
Finally, the researchers dunked the coated particles in a solution that could pass through the titanium dioxide layer and into the sulfur. Once inside, the solution dissolved some of the sulfur and then washed it through the coating. This process washed out enough sulfur to leave room for the fat polysulfides that would form as the battery charged. That means that the battery shouldn’t blow up, says Cui.
A second benefit of the coating: The tiny holes in it are too small to let any dissolved polysulfides escape. That means the battery can stay strong and be recharged more times than batteries made from other compounds.
“The team’s approach has certainly caught the imagination of people,” says Abruña. “This is a nice piece of work,” he adds.
Still, he worries, some problems may remain. For instance, the coating itself fattens the sulfur nanoparticles a little. So it wastes space that could have gone to adding even more energy-holding sulfur. And the air between the particles is an even bigger waste of space. (Think of the space in between individual M&Ms in a jar. If the chocolate weren’t separated into lots of candy-coated pieces, you could fit a lot more chocolate into the jar.)
Nevertheless, tests by Cui’s team show that even after 100 cycles of use and recharging, the new batteries can still hold almost 97 percent of their initial energy capacity. That’s much better, Cui says, than most lithium-ion batteries used in today’s cell phones.
Cui and his coworkers published their findings earlier this year in Nature Communications.







