Cool Jobs: Drilling into the secrets of teeth
Scientists focus on teeth to make better fillings, grow new chompers and explore ancient diets
Researchers are moving well beyond the pick and drill to treat dental problems and “read” the history of our ancient ancestors' health from their not-so-pearly whites.
DragonImages/iStockphoto
Several years ago, Dean Ho bit into a sandwich and chipped a tooth. His dentist patched it up, but several months later the tooth started throbbing. “It was the worst pain I’d ever felt in my life,” Ho recalls.
The inside of the tooth’s root had become infected. Now it needed a root canal treatment. Many patients dread this procedure. It’s where a dentist drills into the tooth and then scrapes out the infected tissue. Afterward, this new hole is filled with a rubber-like material. More than he feared this process, Ho was intrigued by it.
He didn’t understand exactly what a root canal was. That might come as a surprise, since he works at the School of Dentistry at the University of California, Los Angeles. But Ho doesn’t treat patients. He is a bioengineer. Indeed, he heads a lab that uses science and engineering principles to solve problems in biology and medicine.
Reclining in the dental chair, tools and tubes sticking out of his mouth, Ho typed on his smartphone. This curious researcher wanted to “chat” as his dentist set to work. What are you doing? Is it going to hurt? How much longer? When the root-canal treatment was done, Ho had more questions — the kind a bioengineer might ask. Curious about the tooth-filling material, he asked: “Look, is there anything cool about it? Could anything be made better?”
Those timely musings were key. They spurred the creation of a new material that could improve root-canal treatments for generations to come. Ho isn’t the only scientist who has sunk his teeth into … well, teeth! A similar “what if” moment inspired a Boston biologist to try growing new teeth in the lab. And for one archaeologist, bad teeth have turned out to be a boon. They are chock full of DNA — genetic material that offers clues to the diets and diseases of ancient cultures.
A better tooth filling
As Ho learned, infections can be a big and painful problem for root-canal patients. Even after the dentist fills an infected tooth, the seal won’t be perfect. Often it will have little holes. These can allow bacteria to creep in.
Pondering this problem, Ho had an idea. “The wheels were turning while [my dentist] was working on me,” he notes. For years, Ho’s lab had worked with diamonds — not the kind in jewelry, but super-small ones known as nanodiamonds. They’re made from groups of carbon atoms. Each group can be less than a thousandth the width of a human hair. In 2007, his team showed that these teeny pieces of carbon have an unusual blend of flexibility and strength. That suits them for big jobs, such as carrying cell-killing drugs to cancer cells. Since then, Ho’s team has found more uses for nanodiamonds. The material can create sharper medical images and help bone regrow.
At his dentist’s office, Ho had another idea. How about using nanodiamonds to make a better root-canal filler?
Like ordinary diamonds, nanodiamonds are very hard. They can strengthen materials to which they are added. They also are great at latching onto chemicals, including bacteria-killing drugs. These medicines, called antibiotics, can shut down infections. Adding drug-carrying nanodiamonds to the existing filler material could make root-canal treatments more reliable. At the same time, Ho thought, they might keep teeth from getting re-infected.
Back in the lab, he and his coworkers got to work designing this new filler. They compared it to the usual material. For this work, they used teeth that dentists had already extracted from people’s mouths. To fill an infected tooth, dentists need something that’s squishy while it’s being applied, but tough once it hardens. The nanodiamond material looked promising. And lab tests proved it stronger than conventional fillers.
Best yet, this new material indeed can fight infections.
When the researchers spread bacteria onto the two different surfaces, far more bacteria died on the new filler than on the conventional one.
“If the bugs make contact [with the filler], the drug will get them,” Ho says. His team first described this filling material a little more than two years ago in the journal ACS Nano. So far they’ve tried the new filler in three people who needed root canals. When checked six months later, the new material was holding up well and the patients had no further tooth decay. The researchers reported these data in the Nov. 7, 2017 Proceedings of the National Academy of Sciences. Now they’re gearing up for a larger study with 30 people.
Ho also wants his research to motivate “future explorers of the world” — like his preschooler children. “Wouldn’t it be cool if the kids could actually know what I’m doing? There’s impact that can be made beyond the technical side,” he says. Toward that end, Ho has been working with an artist to create a comic book. It not only explains what nanodiamonds are but also how they’re used. (Take a peek at the comics, which you can find at the bottom of this webpage: http://www.projectndx.com/new-page/.)
Lab-grown teeth
Sometimes, teeth rot so much that they cannot be fixed by a root-canal treatment. Instead, dentists have to replace them with dentures or tooth implants. In general, however, such “fake” teeth are far from ideal. “People don’t like them,” notes Pamela Yelick. She’s a biologist at Tufts University School of Dental Medicine in Boston. The reason: Dentures don’t “feel” the same as real teeth do. They also don’t adapt as well to the effects of chewing. Finally, many people find them inconvenient to clean and uncomfortable to wear.
Dental implants have the opposite problem, Yelick adds. Normally teeth attach to soft tissue, called ligaments, which help absorb the forces of chewing. But dentures and implants have no such cushion. That can make fake teeth painful or cause them to break.
Yelick wondered if her team could do a better job. She wanted to find a way to help people grow new teeth.
The idea popped into her head during a lecture by a scientist from the Massachusetts Institute of Technology, in Cambridge. The MIT scientist spoke about his team’s work to engineer artificial livers for kids awaiting a liver transplant.
To grow new livers, the MIT group was using stem cells. Those are rare cells within the human body. Instead of dividing into just one type of cell — such as a skin cell, neuron or bone cell — they can produce any of many different types of cells. Hearing about that research, “I was blown away,” Yelick recalls. “I was like: ‘This is so cool! Could you do this with teeth?’”
To pursue this idea, her team first needed the right stem cells — ones that can give rise to all of the different cell types that make up a tooth. For that, they needed a cheap source of teeth for study. Yelick got creative. She found a pig slaughterhouse that was willing to provide jaws from butchered animals.
Back at the lab, Yelick’s team chiseled out the buds of would-be molars.
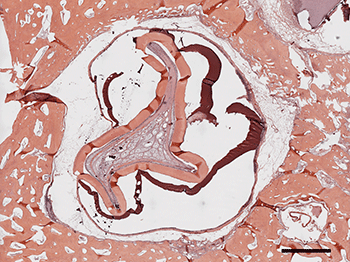
Using existing methods for sorting rare stem cells out of immune-cell mixtures, the researchers isolated stem cells from the pig’s teeth. (Stem cells are identified by a signature collection of proteins on their surface.) The researchers placed the stem cells into dishes filled with a liquid made from cell nutrients. The dishes also contained a three-dimensional mesh known as a scaffold. In several months, those cells grew and organized into “beautiful little tooth crowns,” Yelick says. That was quite a feat. It made the front page of the Boston Globe in 2002.
Since then, Yelick’s team has made tooth crowns starting with dental stem cells from people. They often use wisdom teeth or other teeth extracted by an orthodontist. One day the researchers hope to use this method as an alternative to root canals.
They aren’t there yet. But here’s how they think it could work. Instead of filling damaged teeth with some rubber-like material, they’d apply a gel that contains dental stem cells. Those cells would develop into the various cell types that make up the tooth’s connective tissue. Essentially, Yelick says, this would let stem cells grow on site to replace rotten tissue that had been cleared out during a root canal.
Before anything is done in people, the team needs to do more work in pigs. The pig experiments cost hundreds of thousands of dollars. And these studies would need to be repeated many times to get reliable data. However, Yelick predicts, it should be possible to regenerate human teeth from stem cells within the next 10 years.
Archaeological goldmine
While Ho and Yelick work to save or replace bad teeth, Christina Warinner relishes the rotting chompers. And, she adds, the dirtier, the better!

Warinner works at the University of Oklahoma in Norman. But until 2020, she’ll be a visiting scientist at the Max Planck Institute for the Science of Human History in Jena, Germany. As a molecular anthropologist, she analyzes DNA in ancient bones. Her goal: to learn more about the lives of ancient peoples. Surprisingly, “teeth are one of the best-preserved parts of the skeleton,” she says.
And the most valuable part of ancient teeth? Plaque. “All that stuff getting stuck in your teeth?” It turns rock hard, Warinner says. And that allows it to survive the ravages of time. Materials from saliva cover the tooth with a tough buildup of minerals. As a result, a tooth “doesn’t decompose in the same way as the rest of your body.”
For Warinner, that hardened stuff that dentists scrape off of our teeth holds a remarkable wealth of information. That’s because dental plaque contains loads of DNA from trapped food particles, bacteria in the mouth — even a person’s own cells. By analyzing those bits of genetic information, she and others can learn about what ancient people ate and which diseases they suffered.
The true ‘Paleo’ diet
For example, Warinner’s team discovered that the same microbes that cause gum disease today also troubled ancient peoples. And on the food side, her research has shown that the term “Paleo diet” is misleading. People who follow this modern diet avoid dairy and grains. They eat only fruits, vegetables and other foods that they believe humans consumed during the Paleolithic Era, some 10,000 to 2.6 million years ago.
Books and posters often depict the Paleo diet as one full of bright, healthy foods. “I don’t disagree that eating a breakfast of eggs, avocados and blueberries sounds wonderful,” says Warinner. Alas, her data show, these foods are “brighter, fleshier, sweeter and more calorie-dense than anything a person back then would have had access to.”
Wild avocados of old, for example, had almost no edible fruit — at least not compared to the plump Haas avocados that grocery stores sell today. As for carrots and broccoli, Warinner says, those weren’t even “invented” until the 16th and 17th centuries, after years of plant breeding. Veggies eaten by ancient people were woody, fibrous and tough. Today we would consider these weeds or ornamental plants, says Warinner. Indeed, she adds, “Many people today would not recognize common Paleolithic foods as foods at all.”
As a child, Warinner recalls, someone gave her a book of stamps with Egyptian hieroglyphs (HY-roh-glifs). The ancient world it described enthralled her. A little while later, she got interested in science. The young student had no idea that it was possible to blend elements of history and biology. But that’s what she’s now able to do.
“I loved learning about the natural world, understanding how things work and why they work,” she says. In college, Warinner discovered archaeology. She loved the way that this field could merge with other sciences. “I could look at questions that relate to the human past.” And she could do it, she says, “using tools and techniques coming out of biology and chemistry, which I loved so much.”
Warinner’s studies have taken her all over the globe. She has excavated in the hot, wet Central American jungles of Belize (Beh-LEEZ) and in a beautiful, cold, mountainous region of Mexico.
More recently, Warinner went to Nepal, high in the Himalayas. She traveled with a team of high-altitude archaeologists. They studied ancient people who colonized some of the world’s harshest, high-altitude environments. These sites have few sources of food or other resources.
When a debate arose over which population arrived first, the team settled the issue by reconstructing human genes and more from DNA fragments in excavated bones. Now they’re trying to identify genetic features that helped those ancient people adapt to such cold areas, such intense sun and so little oxygen.
Warinner’s advice to students: “Stay curious and keep your minds open. I never dreamed I could be doing something so much fun!”