Engineers consider liquid salt to generate power
Substance could fuel a new type of nuclear reactor
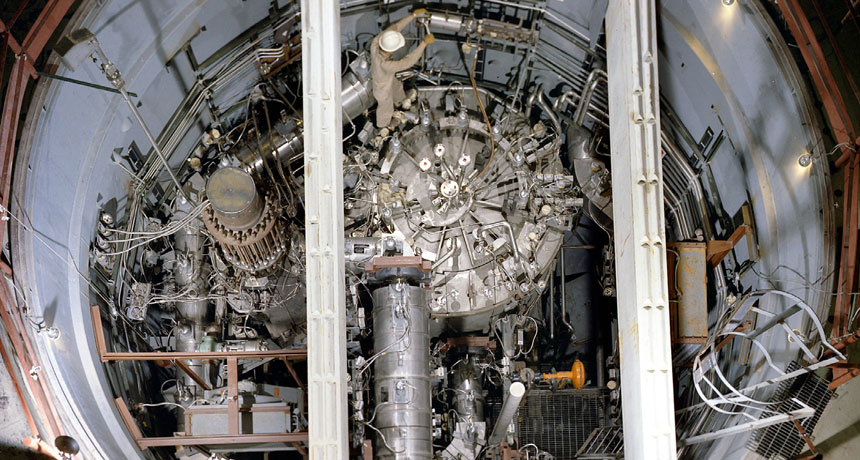
This molten salt nuclear reactor operated in the 1960s. Today, engineers are reviving and improving its design in the hopes of providing affordable power for the future.
Oak Ridge National Laboratory/Wikimedia Commons
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
CAMBRIDGE, Mass. — Radioactive materials make nuclear power possible. These elements, such as uranium, shed energy that can be harvested. But they also are dangerous. They emit radiation. This can harm people and the environment. Leslie Dewan believes, however, that she can reduce risks associated with nuclear power and its radioactive wastes.
She described how at EmTech 2015, a meeting convened here by Technology Review, a magazine of the Massachusetts Institute of Technology.
Dewan is a nuclear engineer and co-founder of Transatomic Power, a local Cambridge-based company. “I became a nuclear engineer because I’m an environmentalist,” she says. Right now, fossil fuels — oil, coal and natural gas — supply more than 80 percent of the world’s energy. But they emit pollution as they burn. They dirty the air and release carbon dioxide, a potent greenhouse gas.
To avoid these problems, people need to switch to cleaner energy sources, Dewan says. These sources include the sun, wind and water. And the switch has to move forward quickly, she and other environmentalists believe.

Indeed, says Jacopo Buongiorno, “The only energy source that allows you to act now is nuclear.” He directs the Center for Advanced Nuclear Energy Systems at MIT and is not involved with Dewan or her company.
The power industry has been hesitant to invest in new nuclear plants. The technology is expensive. But more importantly, there have been several dangerous and costly high-profile accidents.
There was a partial meltdown of fuel during Pennsylvania’s Three Mile Island accident in 1979. The Chernobyl meltdown in Ukraine in 1986 spewed radioactive material broadly. The surrounding countryside remains contaminated today. Then there was the Japanese tsunami that critically damaged reactors at the Fukushima nuclear facility in 2011. That caused some of its nuclear fuel to melt and release radioactive gases into the air.
In light of these events, power companies have been looking for safer options. And Dewan thinks her company has one.
A nuclear ‘Crock-Pot’ with an emergency exit
Dewan’s team is developing a different type of reactor than the ones now operating in most nuclear power plants.
At the core of a nuclear reactor is a radioactive fuel that fissions. That means atoms of the fuel periodically break apart, shooting out particles and energy. Some of those exiting particles may smash into nearby atoms, causing them to fission. If enough of this happens, a self-sustaining chain reaction will occur. This process can produce incredible amounts of energy. Harnessing that energy can heat water to run turbines that produce electricity.
Almost all nuclear reactors now operating are a variety called light water reactors (and yes, there are heavy water reactors too). Solid pellets of uranium, a radioactive material, give off heat in the reactors’ core. These reactors get their name from the fact that water flows through them. This carries away the heat used to make electricity. That heat removal also keeps the reactor fuel from overheating.
Today light water reactors supply almost 5 percent of the world’s energy. But they have two problems. They produce highly radioactive waste that needs to be carefully stored for 300,000 years (a tall order). And they can be very dangerous. If a disaster occurs, the cooling water may escape. If that happens, radioactive material in the reactor can become so hot that it melts and spews dangerous gases into the air. The water also may carry poisons out into the environment. In the Three Mile Island, Chernobyl and Fukushima accidents, reactors lost cooling water. The fuel melted and spewed toxic gases. Nuclear reactors are covered in protective “containment” vessels. But cracks or holes developed in them during these accidents. That allowed radioactive gas to escape into the air.

Dewan and Buongiorno agree that the molten salt reactor should therefore be safer than a light water reactor. But its design never caught on in the 1960s because it was too expensive, Dewan says. Her team now has redesigned the reactor to be smaller and less costly.
This reactor has another advantage, Dewan maintains: It can eat up nuclear waste. A light water reactor uses solid pellets of fuel wrapped in thin metal. Over time, radiation damages that outer coating. The metal also absorbs bits of the atoms as they fission. These pieces may slow down the nuclear chain reaction used to generate energy. So every few years, engineers have to replace the pellets. Those pellets will only have lost 4 percent of their total energy. Still, they must be discarded as waste. Usually this waste then remains at the nuclear power plant. Most plants place that waste in large containers, then submerge those in a pool at the facility.
A molten salt reactor could use the leftover energy in this waste, Dewan explains. The uranium pellets would be dissolved into a liquid salt. This isn’t the same salt you’d shake onto your dinner. Table salt is made of sodium chloride. Dewan’s team plans to use a salt made from the compound lithium fluoride, heated to 600° Celsius (1,112° Fahrenheit).
Without that pesky metal coating, the liquid fuel could stay inside a molten salt reactor much longer, until 96 percent of its energy is gone. As Dewan puts it, the reactor would “simmer like a Crock-Pot.”
Still just an idea
Transatomic Power has not yet built a reactor. Its team has run computer simulations. It’s also testing materials in a lab. Dewan hopes to get started building a prototype by 2020.
Buongiorno cautions that no independent group has yet tested Transatomic’s claims. “Can the reactor do what they’re claiming it can do, in terms of burning nuclear waste?” he asks. “That has not been verified.”
However, he agrees that new reactor designs are important for ensuring that people have enough energy in the future. “Down the road we’re going to need new designs,” he says.
Other groups in the United States, China, and Japan, Dewan notes, are also developing molten salt reactor technology.
Power Words
(for more about Power Words, click here)
atom The basic unit of a chemical element. Atoms are made up of a dense nucleus that contains positively charged protons and neutrally charged neutrons. The nucleus is orbited by a cloud of negatively charged electrons.
carbon dioxide A colorless, odorless gas produced by all animals when the oxygen they inhale reacts with the carbon-rich foods that they’ve eaten. Carbon dioxide also is released when organic matter (including fossil fuels like oil or gas) is burned. Carbon dioxide acts as a greenhouse gas, trapping heat in Earth’s atmosphere. Plants convert carbon dioxide into oxygen during photosynthesis, the process they use to make their own food. The abbreviation for carbon dioxide is CO2.
chain reaction An event that once started continues to keep itself going. It’s a term frequently used to describe atomic fission in a nuclear power plant. When enough fuel is packed closely together, fissioning atoms release neutrons that bombard neighboring atoms, inducing them to fission. This sets up a self-sustaining process.
climate change Long-term, significant change in the climate of Earth. It can happen naturally or in response to human activities, including the burning of fossil fuels and clearing of forests.
element (in chemistry)Each of more than one hundred substances for which the smallest unit of each is a single atom. Examples include hydrogen, oxygen, carbon, lithium and uranium.
environment The sum of all of the things that exist around some organism or the process and the condition those things create for that organism or process. Environment may refer to the weather and ecosystem in which some animal lives, or, perhaps, the temperature, humidity and placement of components in some electronics system or product.
fossil fuel Any fuel — such as coal, petroleum (crude oil) or natural gas — that has developed in the Earth over millions of years from the decayed remains of bacteria, plants or animals.
fission The spontaneous splitting of a large unit into smaller self-sustaining parts. (in physics) A process in which large atomic nuclei break apart to form two or more lighter nuclei. The excess mass of the parent nuclei (compared to the resulting smaller ones) is converted into energy.
greenhouse gas A gas that contributes to the greenhouse effect by absorbing heat. Carbon dioxide is one example of a greenhouse gas.
light water reactor A type of nuclear reactor that runs on solid pellets of radioactive fuel. Water flows through the reactor to remove heat. It turns to steam and then runs through turbines to create electricity. It’s called a “light water” reactor because it uses ordinary water, not water made with a heavy form of hydrogen (deuterium), as some reactors had done.
molten salt reactor A type of nuclear reactor that runs on a liquid, radioactive fuel. That fuel consists of a solution of enriched uranium (and sometimes thorium) dissolved into a liquid salt. The liquid moves within a pipe that travels through the reactor, where it picks up heat. The pipe then leaves the reactor, passes through water, which picks up some of the heat and turns it to steam. This steam is then used to turn turbines that generate electricity.
natural gas A mix of gases that developed underground over millions of years (often in association with crude oil). Most natural gas starts out as 50 to 90 percent methane, along with small amounts of heavier hydrocarbons, such as propane and butane.
nuclear engineer Someone who tests, builds or runs nuclear reactors or nuclear power technology.
nuclear power Energy derived from processes that produce heat by splitting apart the nuclei of atoms (fission) or forcing atomic nuclei to merge (fusion). A nuclear power plant uses that heat to drive turbines that create electricity.
nuclear reactor A large machine in which radioactive fuel undergoes a steady chain of fission reactions, which generate heat.
nuclear waste Fuel that has been through a nuclear reactor. Typically, it is still radioactive and dangerous.
particle A minute amount of something.
prototype A first or early model of some device, system or product that still needs to be perfected.
radioactive An adjective that describes unstable elements, such as certain forms (isotopes) of uranium and plutonium. Such elements are said to be unstable because their nucleus sheds energy that is carried away by photons and/or and often one or more subatomic particles. This emission of energy is by a process known as radioactive decay.
salt A compound made by combining an acid with a base (in a reaction that also creates water).
sodium A soft, silvery metallic element that will interact explosively when added to water. It is also a basic building block of table salt (a molecule of which consists of one atom of sodium and one atom of chlorine: NaCl).
solar Having to do with the sun, including the light and energy it gives off.
technology The application of scientific knowledge for practical purposes, especially in industry — or the devices, processes and systems that result from those efforts.
uranium The largest naturally occurring element known. It’s called element 92, which refers to the number of protons in its nucleus. One form (isotope) is radioactive, which means it decays into smaller particles. The other form is stable.







