Build ice towers with bottled water and ice
Add cold water and salt to create the conditions for your own mini ice castle

Bottles of water can be poured to create a tower of solid ice if the water is cold enough.
Doram/iStock/Getty Images Plus
This article is one of a series of Experiments meant to teach students about how science is done, from generating a hypothesis and designing an experiment to analyzing the results with statistics. You can repeat the steps here and compare your results — or use this as inspiration to design your own experiment.
In the Disney movies Frozen and Frozen II, Elsa uses magical powers to make castles of ice. We can’t build castles, but we can pour out small ice towers. Elsa may have magic. We use science.
In an earlier experiment, I showed that sugar seed crystals are important for making rock candy. Those crystals provide a nucleation point where the sugar structure — the candy — can self-assemble. But sugar isn’t the only substance that can form a structure with the right nucleation point. Water can, too. It just needs to be super cool.
If you pour extremely cold water (water that’s less than 0° Celsius or 32° Fahrenheit) onto an ice cube, that cube acts as a nucleation point. The water will freeze when it hits the cube, and then stack up — forming a tower of ice.
If the water isn’t cold enough, though, you just get a lonely ice cube in a puddle of water.
As water freezes at 0 °C, I need a way to chill water to cooler than that. And I can’t let it freeze until it comes into contact with the cube. How? The secret is salt.
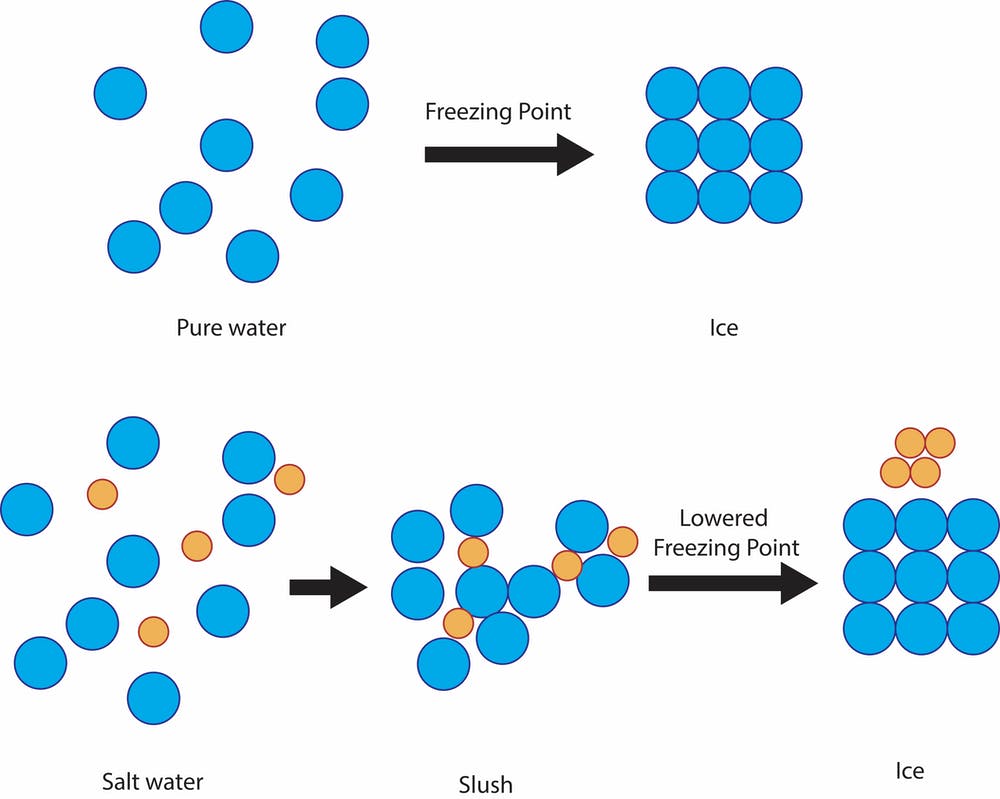
Saltwater has a lower freezing point than freshwater. This is why you can spread salt on an icy sidewalk and the ice will melt. Salt crystals in the water prevent the water molecules from linking up to form a crystal. Saltwater needs to be even colder than freshwater to freeze — cold enough that the salt gets pushed out of the water’s crystal structure. Freshwater freezes at 0 °C (32 °F), but saltwater freezes at –2 °C (28.4 °F).
Is salt the difference between an ice tower and a puddle? I’m going to do an experiment to find out.
Chill out
I need to start with a hypothesis — a statement I can test. Here, my hypothesis is: Water chilled in ice with salt will be colder and form taller ice towers than water chilled in water without salt.

To test this, I start with two small coolers. One cooler just has ice. The second cooler has ice mixed into a slush with salt.
I place bottles of purified water into each cooler. Be careful to check the label when you buy the water. The water should be purified or distilled. It should contain no added minerals. Those minerals might serve as nucleation points — making the bottled water freeze into a solid block of ice as soon as it gets cold enough to freeze. But if the water is pure, it should be able to get colder than 0 °C (32 °F) and stay a liquid.
Just one bottle of water in each cooler isn’t enough. I want to make sure that I can collect enough data to detect a difference between water chilled in fresh or salty ice. So I put eight bottles of water into each cooler.
Here’s how how to build ice towers:
- Fill two smaller coolers with ice (or you can use one cooler, and run the experiment twice).
- In one cooler, add 700 grams of salt (24.7 ounces, or about 2.5 cups). With a spoon, mix the salt and ice together very well. The ice will immediately start melting into a thick slush.
- Sink eight bottles of purified water into each cooler (for a total of 16). Make sure the bottles are each evenly surrounded with ice or slush.
- Wait 25 minutes.
- Gently rotate the bottles in the ice or slush. Make sure you do not shake the bottles.
- Wait another 20 minutes.
- Take the temperature of the bottles. I did this with a no-contact thermometer, but you can also use a regular thermometer; sink it into the ice next to the bottles.
- Place an ice cube on a plate. Using a ruler, measure the height of the cube.
- Remove a bottle of water carefully from the ice or slush and open it. Again, be careful not to shake it. (If you shake the super-cooled water, air inside the bottle will mix with the water. That creates nucleation points — and your bottled water will freeze before you open it.)
- Gently and slowly pour a small, steady stream of water onto the ice cube. After a second or two, the water will begin to freeze on top of the cube, producing an ice tower.
- Quickly whip out your ruler and get the height of your ice tower before it melts. (A friend is helpful for this.)
- Repeat until you’ve tested all 16 of your bottles.

Add salt to your ice, and stir until you get a chilly slushB. Brookshire 
Sink your water bottles into the ice and wait. After 45 minutes, take the temperature of the water bottles. B. Brookshire 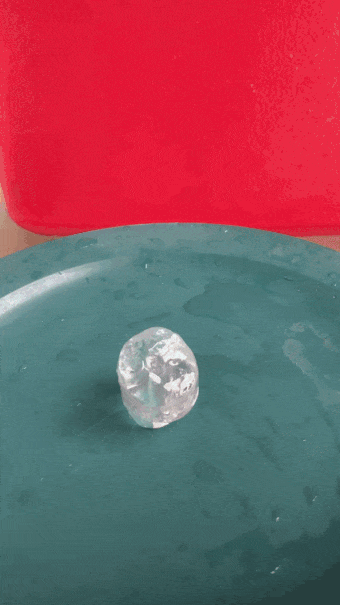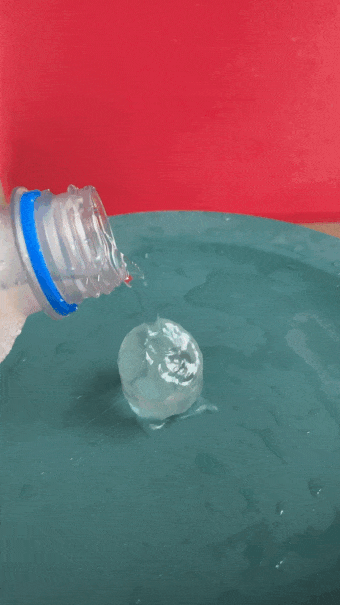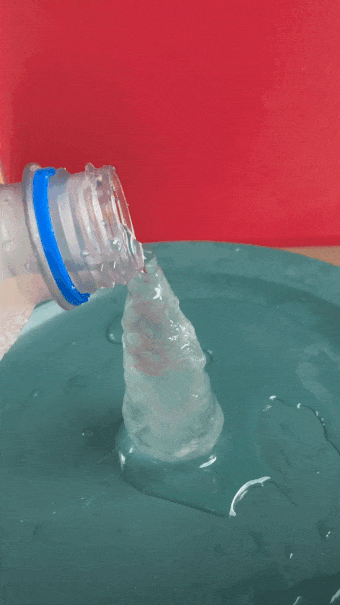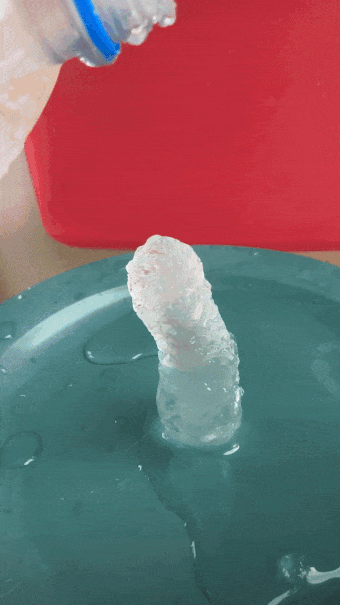
When the water is super cold, pour slowly and carefully. An ice tower will grow. B. Brookshire
Results on ice
To calculate how much ice I was able to grow, I subtracted the height of the ice cube from the height of the final ice tower. I added that to a spreadsheet with the temperature of each ice bottle.
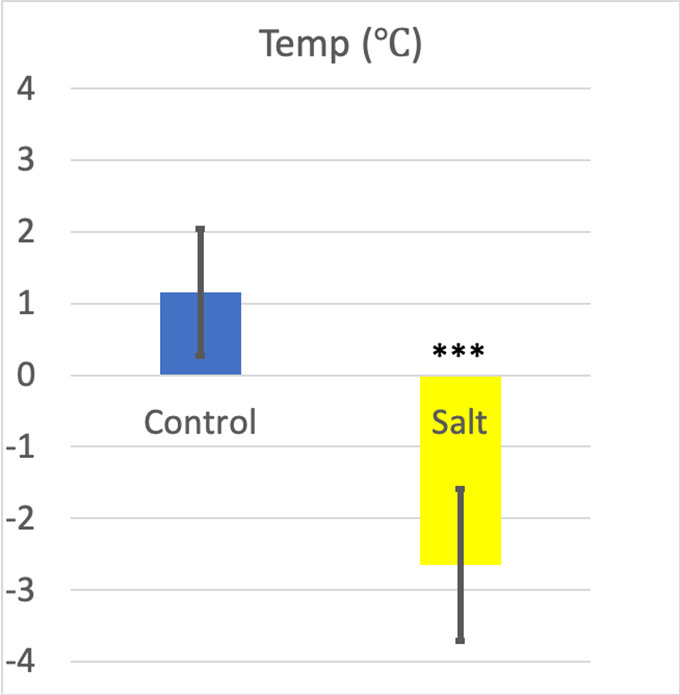
The bottles chilled in ice got pretty cold — an average of 1.16 °C (34.1 °F). But the bottles chilled in salt slush got colder — an average of –2.65 °C (27.2 °F).

To confirm that these two groups are indeed different, I need to run statistics. These are tests to interpret the meaning of my results. I ran a t test. This test finds differences between two groups. Lots of websites online will let you plug your data in to run these tests for free. I used one from GraphPad Prism.
The test gave me a p value. It’s a measure of how likely it is that I would by accident find a difference as big as the one I found here. Many scientists consider a p value of less than five percent (0.05) to be statistically significant. This means that I would have a five percent chance of finding a difference that size by accident alone. If the p value I get is less than 0.05, I have a statistically significant difference.
For the two temperatures in my experiment, the p value was 0.0001, or 0.01 percent — definitely significant.
But just because there is a difference doesn’t mean that this difference is large. Tiny differences can be statistically significant. So I also ran a Cohen’s d test. Here I had to calculate my standard deviation. This is the amount that each set of data differs from the mean (or average). You can calculate that in any spreadsheet on a computer. On mine, I used Microsoft Excel, and used the function “= STDEV” and highlighted my data set. Then I put my mean, standard deviation and number of samples into this online calculator.
My Cohen’s d result was 2.93. Many scientists consider a Cohen’s d higher than 0.8 as a large effect size. So, the water cooled in the salt slush was definitely colder than the water cooled in simple ice.
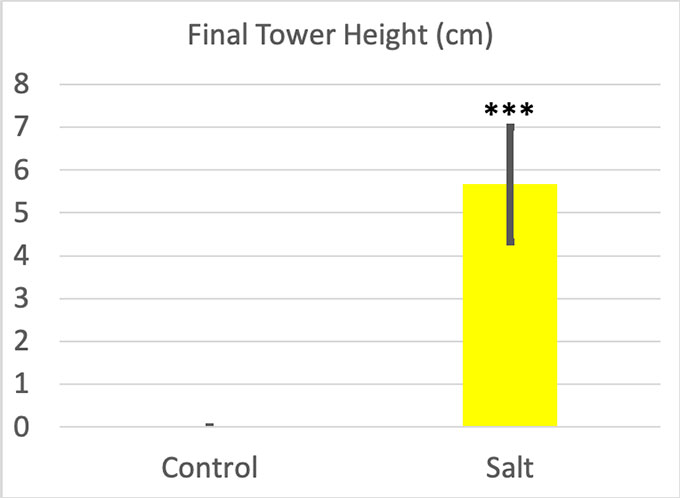
As for the towers I built, the difference looked pretty obvious. When I poured out the bottles chilled in clean ice, I just got a puddle of water around my ice cube. Not a single one grew a tower. The difference between the ice cube and the final tower height was zero every time. But my saltwater-chilled bottles produced visible towers. Their average height was 5.66 centimeters (2.22 inches).
I ran statistics for these results as well. The p value for my final towers was 0.0001. My Cohen’s d was 4.13. Salt water made a very big difference in tower height.
My hypothesis when I started was that water chilled in ice with salt will be colder and form taller ice towers than water chilled in water without salt. Based on my results, it seems I was right. Salt helped water get colder and grow taller towers.
In every experiment, there are always things I could have done differently. In this one, I ended up with only six usable saltwater-chilled water bottles. The other two froze. Water in those bottles may not have been quite as “pure” as advertised on the label. I would probably chill extra bottles next time. Not only that, I didn’t always pour out the exact same quantity of water. Next time, I could weigh my results, and make sure that I poured out the same mass of water each time. That might help me get even more accurate results.
Luckily, this experiment is cheap. And I can easily run it again.
Materials:
- Ice (two bags): $1.99 each
- Purified water (two packs of eight): $3.28 each
- 9-quart cooler (two): $9.97 each
- Salt (three pound box): $2.63
- Handheld infrared thermometer: $18.00
- Ruler: $0.97







