Experiment: Test the effect of temperature on reaction time
Can you make an Alka-Seltzer tablet dropped in water fizzle faster or more loudly by changing the water’s temperature?

Figure 1. In this experiment, we investigate how to make Alka-Seltzer tablets plunked in water fizzle faster and more furiously.
asadykov/iStock/Getty Images Plus
Objective: To measure the effect of temperature on the rate of a chemical reaction
Areas of science: Chemistry, science with your smartphone
Difficulty: Easy intermediate
Time required: 2–5 days
Prerequisites: None
Material availability: Readily available
Cost: Very low (under $20)
Safety: Adult supervision may be needed when working with hot water solutions
Credits: Andrew Olson, PhD, Science Buddies; edited by Svenja Lohner, PhD, Science Buddies
You may have seen a television commercial for Alka-Seltzer tablets or heard one of their advertising slogans: “Plop, plop, fizz, fizz, oh what a relief it is!” When you drop the tablets in water, they make a lot of bubbles, like an extra-fizzy soda, as shown in the main image up top (Figure 1). And like a soda, the bubbles are carbon dioxide gas (CO2). However, with Alka-Seltzer, the CO2 is produced by a chemical reaction that occurs when the tablets dissolve in water.
Alka-Seltzer is a medical drug that works as a pain reliever and an antacid (antacids help neutralize stomach acidity, such as heartburn). The pain reliever used is aspirin and the antacid used is baking soda (sodium bicarbonate, NaHCO3). To take the tablets, they should be fully dissolved in a glass of water. When sodium bicarbonate dissolves in water, it dissociates (splits apart) into sodium (Na+) and bicarbonate (HCO3) ions. (An ion is a molecule that has a charge, either positive or negative.) The bicarbonate reacts with hydrogen ions (H+) from citric acid (another ingredient in the tablets) to form carbon dioxide gas and water. In other words, carbon dioxide gas is a product of this reaction. The reaction is described by Equation 1 below:
Equation 1.
3HCO3− + 3H+ → 3H2O + 3CO2
So how is temperature related to this bicarbonate reaction? In order for the reaction shown above to occur, the bicarbonate ions have to come into contact with the hydrogen ions. Molecules in a solution are in constant motion and are constantly colliding with one another. The hydrogen and bicarbonate ions must collide at the right angle and with enough energy for the reaction to occur. The temperature of a solution is a measure of the average motion (kinetic energy) of the molecules in the solution. The higher the temperature, the faster the molecules are moving. What effect do you think temperature will have on the speed, or rate, of the bicarbonate reaction?
In this chemistry science project, you will find out for yourself by plopping Alka-Seltzer tablets into water at different temperatures and measuring how long it takes for the chemical reaction to go to completion. In addition, you can record the sound of the Alka-Seltzer fizzle using a smartphone equipped with a sensor app. Do you think it will fizz more loudly in hot or cold water?
Terms and Concepts
- Chemical reaction
- Alka-Seltzer
- Baking soda, or sodium bicarbonate
- Molecule
- Products
- Temperature
- Bicarbonate reaction
- Reaction rate
Questions
- What is the bicarbonate reaction? What are its products?
- Keeping in mind that an increase in temperature reflects an increase in the average molecular motion, how do you think increasing temperature will affect the reaction rate?
- What temperature change do you think would be required to increase, or decrease, the reaction time by a factor of two?
- What other factors besides temperature can affect how well a chemical reaction takes place?
Materials and Equipment
- Alka-Seltzer tablets (at least 12; if you plan to do additional variations to the project, you will want to get a larger box)
- Thermometer with a range of at least 0°C to 60°C (32°F to 140°F)
- A suitable thermometer is available from Amazon.com
- A standard kitchen candy thermometer will also work fine
- Clear drinking glasses or jars; about 8 ounces, or 240 milliliters (two of the same size)
- Graduated cylinder, 100 mL. A 100 mL graduated cylinder is available from Amazon.com. Alternatively, measuring cups may be used.
- Masking tape
- Hot and cold tap water
- Ice
- With option 2 in procedure: Stopwatch or a clock or watch with a second hand
- Optional: A helper
- Lab notebook
- Pencil
- With option 1 in procedure: Smartphone with a sensor app such as phyphox, available for free on Google Play for Android devices (version 4.0 or newer) or from the App Store for iOS devices (iOS 9.0 or newer).
- With option 1 in procedure: Small sealable (waterproof) plastic bag that fits your phone inside of it
| Condition | Temperature (°C) | Reaction Time (s) | Optional: Maximum Sound Intensity (dB) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial #1 | Trial #2 | Trial #3 | Average | Trial #1 | Trial #2 | Trial #3 | Average | ||
| Hot Tap Water | |||||||||
| Cold Tap Water | |||||||||
| Ice Water | |||||||||
Experimental Procedure
Note: In this science project, you will investigate how water temperature affects the dissolving time of an Alka-Seltzer tablet. You will use a smartphone equipped with a sensor app to record the fizzing sound of the Alka-Seltzer reaction in water and measure the time it takes for one Alka-Seltzer tablet to react completely in water. The app creates a graph that will not only give you information about the reaction time but will also allow you to assess how loud each reaction was based on the measured sound intensities. If you do not have a phone, you can observe the reaction and use a stopwatch to time how long it takes for each tablet to dissolve.

- Do your background research and make sure that you are familiar with the terms and concepts in the Background.
- In your lab notebook, make a data table like Table 1. You will record your results in this data table.
- Prepare a drinking glass so that it is marked at the 200 mL point. You will use the same glass for multiple trials, so it is convenient to mark the desired water level. Note: If your glass fits more than 8 ounces, make a mark about 1 inch below the rim.
- Add 200 mL (a little less than 1 cup) of water to the drinking glass, or fill it up to about 1 inch below the rim.
- Use a piece of masking tape on the outside of the glass to mark the water level, placing the tape with its top edge even with the water level in the glass, as shown in Figure 2.
- Note: You do not want to fill the glass completely full because the bicarbonate reaction produces bubbles that could splash out.
- You will fill the drinking glass with the same volume of water at three different temperatures: hot tap water, cold tap water and ice water.
- For the hot and cold tap water, run the water until the temperature stabilizes. Fill the glass with water to the level of the masking tape. Be careful when handling the hot water.
- For ice water, fill the glass about half full with ice cubes, then add cold tap water to a bit above the level of the masking tape. Stir for a minute or two so that the temperature equilibrates. Once temperature has equilibrated, remove the ice cubes from the water’s surface using a spoon or other utensil immediately before adding the Alka-Seltzer tablet. (Pour out any extra water so that the water is up to the level of the masking tape.)
- Prepare the drinking glass with one of the three temperatures as described in step 4. Then measure the reaction time for that temperature either by following option 1 (sensor app), described in step 6, or option 2 (stopwatch), described in step 7.
If you use the phyphox app to measure the amplitude of sounds, you will need to calibrate the sensor first to get correct decibel readings on your device. The sensor has to be recalibrated between individual recordings. Instructions on how to do the phyphox sound sensor calibration are provided in the video above. - Option 1: Using the Sensor App
Sensor apps such as phyphox let you record data using sensors that are built into many smartphones, including a microphone that you can use to measure sound. In this project, you can use the app to record the fizzing sound that the Alka-Seltzer tablet makes while it dissolves in water and then use the data to determine the reaction time and maximum sound intensity for each reaction.- Open the sensor app on your phone and select the sound sensor (audio amplitude in phyphox). Remember, that when you are using the phyphox app you will have to calibrate the audio amplitude sensor (sound sensor) before you do any measurements. Do this calibration before you start your investigation, so you get correct sound intensity readings. To calibrate your sound sensor in phyphox, follow the instructions in the sound sensor calibration video. You will have to re-calibrate the audio amplitude sensor (re-set the decibel offset) every time you start a new recording! Once you have calibrated the sensor, make sure you know where the microphone is located on your phone and do a quick test to see if your sound measurement is working. For example, you could record yourself clapping or singing to check if the sensor behaves as expected.
- Once you have confirmed that the sensor works and you are familiar with the app, you can start with the experiment. You should do this experiment in a quiet environment. The background reading of your sound meter when there is no noise in the room should be in the range between 20–40 decibels (dB).
- Measure the temperature of the water (in Celsius [C]) in the first glass that you prepared, and record it in the data table in your lab notebook. Remove the thermometer from the glass before continuing with the next step.
- Put your phone in the waterproof plastic bag and make sure it is sealed well. You don’t want it to get wet!
- Place the second, same-sized glass, next to the glass filled with water. Lay your phone on top of the second glass so that the microphone (or sound sensor) is located right at the center above the glass filled with water, as shown in Figure 3.

Figure 3. Place your phone on top of the glass filled with water so that the microphone (or sound sensor) is located right at the center above the solution.M. Temming
- Take one whole Alka-Seltzer tablet out of its package and hold it above the glass filled with water. In the phyphox app, start a new recording for your first experiment by pressing the play button.
- Once the recording starts, drop the tablet into the water. Note: You have to be very quiet during the experiment. Any sound that you make will be recorded and could affect your data. Try to be as quiet as possible while you are recording your data!
- You will immediately see and hear bubbles of CO2 streaming out from the tablet.
- The tablet will gradually disintegrate. Observe the graph recorded by the app, and how the sound sensor is responding to the fizzling while all of the solid white material from the tablet disappears.
- When the solid material has completely disappeared, and you see on the graph that the sound intensity has reached background levels again or does not change anymore, wait 20 more seconds until all the bubbles have stopped forming, and stop recording your data. Make sure to save your data and label it appropriately such as “hot water,” “cold water” or “ice water.”
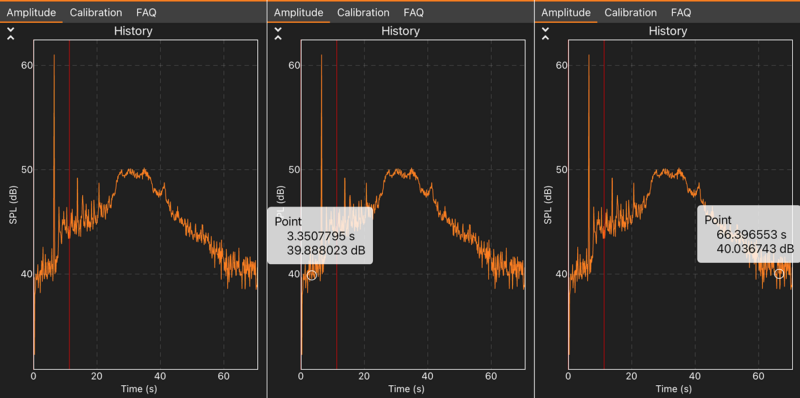
Figure 4. This example data from the phyphox app demonstrates how to measure the reaction time of the Alka-Seltzer tablet dissolving. The x-axes of the graphs are time in seconds [s] and the y-axes shows sound intensity in decibels [dB].Made with phyphox by M. Temming
- Your data should look something like the graph in Figure 4. Your graph should show an increased sound intensity for as long as the Alka-Seltzer reaction took place. The sound level of the reaction might be louder in the beginning and decrease as the tablet gets smaller. In the graph, every bubble that pops in the solution is represented by a spike.
- Measure the time between the beginning of your reaction (when you dropped the tablet and the sound intensity started to increase) and the end of the reaction (when the sound intensity reached background levels again or does not change significantly anymore). In phyphox, you can use the “pick data” function to select the respective data points and view their time and decibel values. For example, the reaction in Figure 4 started a little after 3 seconds and ended at about 66 seconds.
- Calculate the time difference between these two points. The result is the reaction time for your first trial. Record the reaction time (in seconds [s]) in the data table in your lab notebook.
- Tip: Be careful when opening the packets and handling the Alka-Seltzer tablets. The tablets are thin and brittle, so they break easily. If some of the tablets are whole, and some are broken into many pieces, the separate trials will not be a fair test. You should only use whole tablets.
- Option 2: Using the stopwatch
- After filling the glass to the level of the masking tape, measure the temperature of the water (in Celsius [C]), and record it in the data table in your lab notebook.
- Remove the thermometer from the glass before continuing with the next step.
- Have your helper get ready with the stop watch, while you get ready with an Alka-Seltzer tablet. Have your helper count one–two–three. On three, the helper starts the stop watch and you drop the tablet into the water.
- You will immediately see bubbles of CO2 streaming out from the tablet.
- The tablet will gradually disintegrate. Watch for all of the solid white material from the tablet to disappear.
- When the solid material has completely disappeared, and the bubbles have stopped forming, say “Stop!” to have your helper stop the stopwatch.
- Record the reaction time (in seconds [s]) in the data table in your lab notebook.
- Tip: Be careful when opening the packets and handling the Alka-Seltzer tablets. The tablets are thin and brittle, so they break easily. If some of the tablets are whole, and some are broken into many pieces, the separate trials will not be a fair test. You should only use whole tablets.
- Repeat step 6 or 7 two more times with the same temperature of water. If you use the sensor app, make sure your sound sensor is still calibrated and recalibrate it again (re-set the decibel offset) if necessary before each recording.
- Repeating an experiment helps ensure that your results are accurate and reproducible.
- Repeat steps 5 and 6 or 5 and 7 for each of the other temperatures.
- When you are done, you should have done a total of three trials for each of the three temperatures.
- Calculate the average reaction time for each of the three water temperatures. Record your results in the data table in your lab notebook.
- Make a graph of the average reaction time, in seconds (on the Y-axis), vs. water temperature, in degrees Celsius (on the X-axis).
- How does reaction time change with temperature? Can you explain why this is?
- Hint: If you are having trouble explaining your results, try re-reading the Introduction in the Background.
- If you chose to use a sensor app to record your data, look at the graphs for each water temperature again. Write down the maximum sound intensity that you observed during the Alka-Seltzer reaction (not including the initial or end peaks) for each trial. You can get the number in the phyphox app by using the “pick data” tool to select the timepoint at which the sound intensity is highest. In the example shown in Figure 4, this would be around 35 seconds with a sound intensity of about 50 decibels. Calculate the average for each of the three water temperatures and record your results in the data table in your lab notebook.
- Make a graph of the average maximum sound intensity, in decibels (on the Y-axis), vs. water temperature, in degree Celsius (on the X-axis).
- Which reaction was the loudest? Did you expect these results?
Variations
- More advanced students should also calculate the standard deviation of the reaction times for each temperature.
- Use the standard deviation to add error bars to your graph.
- For example, say that the average reaction time for one temperature was 45 seconds, and the standard deviation was 5.2 seconds (these are made-up numbers). You would graph the symbol for the data point at 45 seconds, and then draw short vertical bars above and below the symbol. Each vertical bar would have a length equivalent to 5.2 seconds.
- Error bars give your audience a measure of the variance in your data.
- Adult supervision required. Is reaction rate predictable over a larger temperature range? Water remains liquid above 0° C and below 100° C. Repeat the experiment at one or more additional high temperatures to find out. Use Pyrex glass for containing water heated on the stove or in the microwave, and use appropriate care (e.g., wear hot mitts and safety goggles) when handling hot water. A standard candy thermometer should be able to measure the temperatures in this higher range.
- You could turn the bicarbonate reaction into a home-made lava lamp. To do this, you will want to use a tall jar or empty clear plastic 1-liter or 2-liter bottle, fill it with 2 to 5 centimeters (cm) of water, add 5 drops of food coloring, and then fill it at least three-quarters full with vegetable oil. You could repeat the science project using your homemade lava lamp at a cold and a hot temperature. To do this, you will need to figure out a way to make the prepared bottle hot or cold. (For example, to make it hot you could let it sit in a large bowl of hot water, and to make it cold you could store it in a refrigerator or freezer.) You will also want to use one-quarter of an Alka-Seltzer tablet at a time (instead of a whole tablet). How does the bicarbonate reaction look and function in the home-made lava lamp?
This activity is brought to you in partnership with Science Buddies. Find the original activity on the Science Buddies website.








