Explainer: Ions and radicals in our world
When atoms get an electric charge, they act very differently and take on a new name

The citric acid present in lemon juice gets its puckering bite from hydrogen ions.
BSIP/UIG/Getty Images
Atoms are the smallest particles with distinct chemical properties. Each is made of at least two types of smaller particles: protons and electrons. Protons have a positive electrical charge. Electrons are negatively charged. Atoms of the same element always have equal numbers of protons and electrons. That balance makes the atoms electrically neutral.
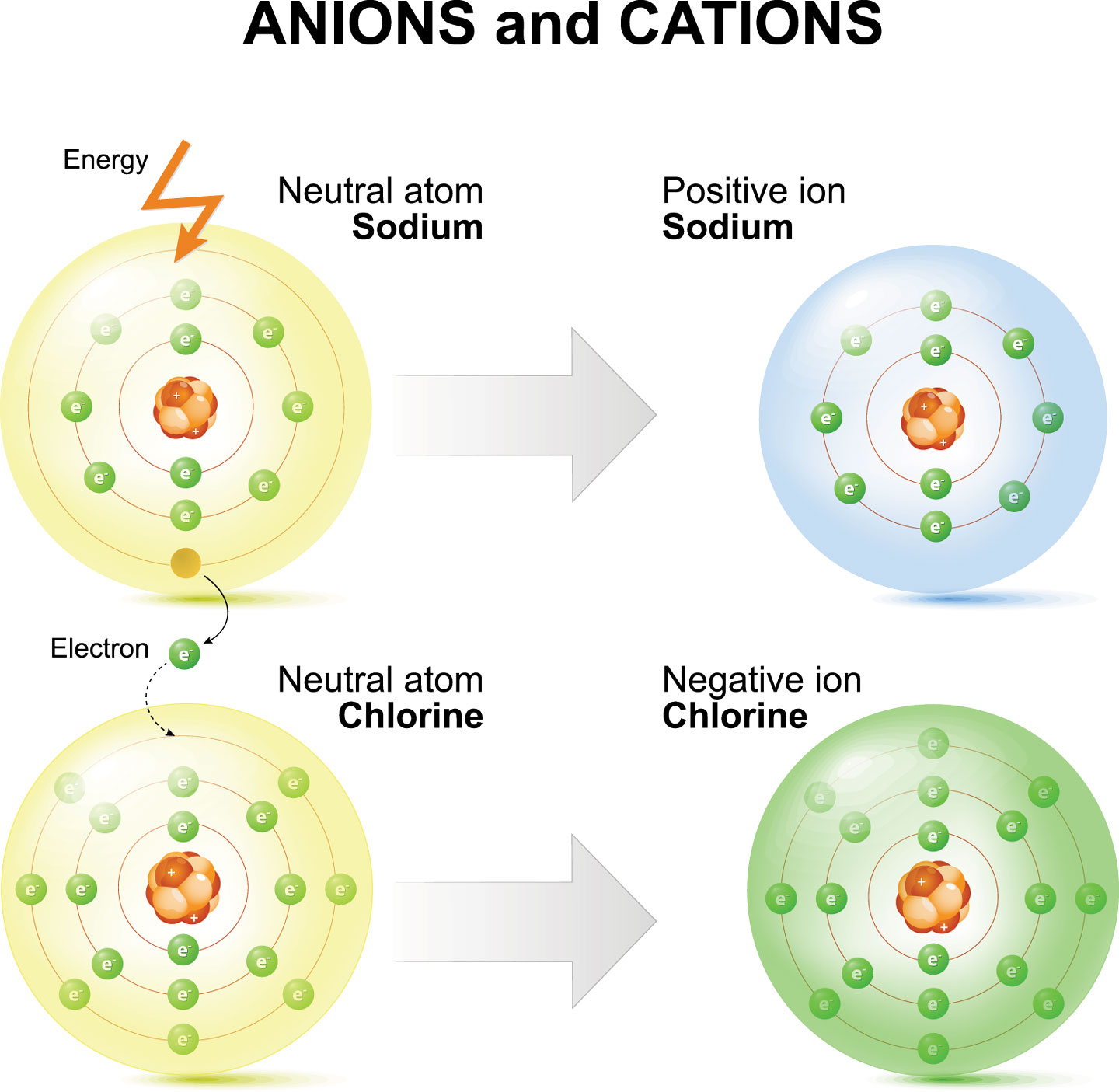
But chemical reactions sometimes throw off that electrical balance by robbing one or more of an atom’s electrons or by getting an atom to hold one or more extra electrons. Because the number of protons hasn’t changed, the atom is no longer electrically neutral. It’s charged. Such “charged atoms” are known as ions. Some ions get slightly modified names when compared to their former status as an atom.
Ions come in two types. Atoms that gain electrons are known as anions (AN-eye-uns). These have an overall negative charge. The size of that charge depends on how many electrons it gained.
For example, an oxygen atom starts with eight protons and eight electrons. It is given the symbol O. When it forms its most common ion, oxygen gains two electrons to become the “oxide anion.” Chemists denote this by adding a 2-minus on the upper right side (O2-). That superscript tells you its charge.
Atoms that lose electrons become cations (CAT-eye-uns). These have an overall positive charge. For example, an aluminum atom (symbol Al) starts with 13 protons and 13 electrons. When the atom forms its most common ion, it loses three electrons, becoming the cation Al3+.
In general, metal atoms tend to lose electrons to form cations. Non-metal atoms tend to gain electrons and form anions. This means metals often react with non-metals, releasing electrons to them. This process immediately makes ions of both the electron donor and the receiving atom. Once this happens, an electrical attraction develops between these positive and negative ions. It’s called a Coulombic attraction or force. You may know it simply as “opposites attract.”
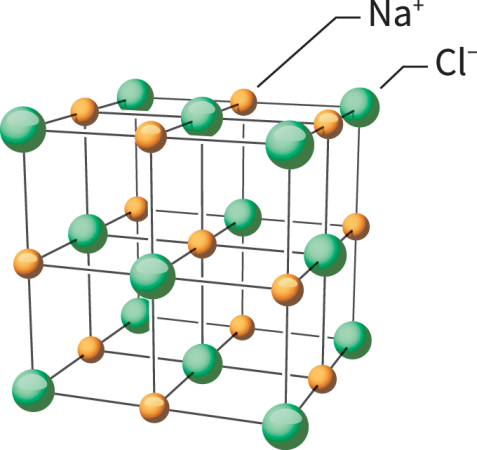
Chemical bonds forged by this attraction are ionic bonds. Bonded cations and anions make up ionic compounds.
The most well-known example of an ionic compound is table salt. One sodium atom (Na) gives a single electron to a chlorine atom (Cl). This forms Na+ and Cl– ions. (No number appears with the superscript when an atom gains or loses a single electron.) The ionic compound formed between sodium and chloride ions is called sodium chloride. Ionic bonds are strong. This means that sodium chloride has a high melting and boiling point. It also is brittle. Salt’s ions will collect to form a giant, three-dimensional structure known as a lattice.
The ions’ charges also attract salt to water. Water does not contain ions. But it does have tiny positive and negative electrical charges on its surface. These tiny charges attract positive and negative ions. That allows table salt to dissolve easily in water.
A major player in chemistry
Ions have many important roles to play. For example, they carry the charges in batteries, conducting electricity. Hydrogen ions, H+, also define an acid. Any substance that produces these ions is said to be acidic.
The chemical process where electrons are lost and gained is called REDOX. It’s a mashup of REDuction and OXidation. In terms of electron transfer, reduction is defined as a gain of electrons. Oxidation is a loss of electrons. We can write two equations, called half equations, to show each half of that REDOX process.
For example, here they are for sodium chloride,
OXIDATION (electron loss): Na → Na+ + e–
REDUCTION (electron gain): Cl + e– → Cl–
Reduction and oxidation always take place at the same time. It’s impossible, after all, for one atom to gain an electron unless another atom is losing one.
Radicals
Electrons tend to come in pairs. A lone electron in an atom or molecule makes that atom or molecule very unstable. Here, unstable means very chemically reactive. They now are inclined to undergo chemical reactions with other atoms or molecules.
Unpaired electrons are looking to pair up with others. Atoms or molecules with unpaired electrons are called radicals. Radicals are illustrated in chemistry by using a dot. For example, Cl· represents a chlorine radical.
Radicals’ high reactivity can cause problems. They can damage human cells. And in the environment, radicals can react with ozone in Earth’s stratosphere. Ozone is a molecule consisting of three bound oxygen atoms (O3). This ozone normally protects the planet from the sun’s harmful ultraviolet radiation.
But when chlorine radicals react with ozone, the O3 is broken down into molecular oxygen (O2) and a chlorine monoxide radical. What’s left are ‘holes’ — or a thinning — in the protective ozone layer. That can let more of the sun’s harmful ultraviolet rays reach Earth’s surface.
In the first reaction, chlorine radicals react with ozone to convert it to oxygen.
Cl• + O3 → O2 + ClO•
In the second reaction, ClO radicals produce more Cl radicals. Then, the reaction above starts again. ClO• + O → Cl• + O2
This chain reaction is very damaging since it cannot easily be stopped. The only reaction that breaks the cycle is a pairing of the radicals. For example, Cl• + Cl• → Cl2. This sometimes happens when two radicals collide.
The source of many harmful chlorine radicals in the atmosphere were refrigerants used in air conditioning and aerosol cans. The good news: Those chemicals were eventually banned. This has allowed the ozone layer to begin to heal.







