Explainer: Ocean acidification
Here’s what happens when seawater is forced to absorb too much carbon dioxide
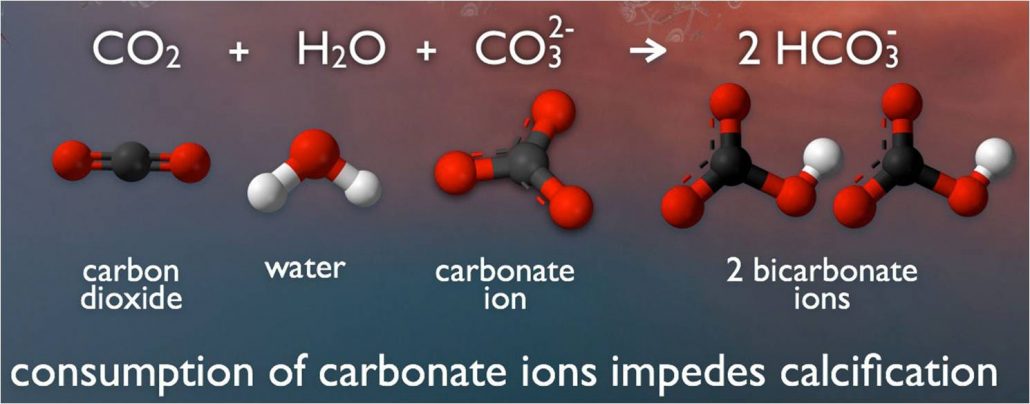
When carbon dioxide dissolves in ocean water, a process begins that uses up carbonate molecules. So more carbon dioxide means less carbonates for shell building by swimming snails and other creatures.
NOAA
Oceans cover 72 percent of our planet. Every day they remove about 22 million tons of carbon dioxide from the air. That’s helpful since too much of this carbon dioxide — a gas known as CO2 — will cause Earth’s atmosphere to become overly warm. That process is already underway. Climate scientists attribute this global warming to the greenhouse effect. And that explains why scientists refer to carbon dioxide, or CO2, as a greenhouse gas.
Of every 10 tons of CO2 added to the atmosphere, two or three tons end up in the water. And as human activities have been adding more CO2 to the air, the chemistry of the oceans has been changing. They are becoming more acidic through a process called ocean acidification.
Acids include liquids that taste sour, such as vinegar and lemon juice. These materials react with bases ― substances, such as ammonia or lye, that feel slippery — to form salts. Distilled water is neutral, which means it’s neither an acid nor a base. Scientists measure acidity using the pH scale. Acids have a pH between 0 and 7. Bases have a pH between 7 and 14. (Neutral water has a pH of 7.0.)
Ocean water tends to be slightly basic. It has a pH of about 8.1. But that number has been falling ― turning more acidic ― as the amount of CO2 in ocean water has been rising. Seawater is not yet acidic. But it is moving in the direction of becoming an acid. That’s happening, and it’s happening fast.
By 2100, if people are still adding the same amount of CO2 into the air as they are today, the oceans will be more than twice as acidic as they were before the Industrial Revolution. (The Industrial Revolution began more than 200 years ago and describes the rapid growth of industry.)
“We don’t really give it much thought,” says Peter Neill, “but [ocean acidification] may be one of the most important aspects of what’s going on [with climate change]. And it may be the one that lasts the longest.” Neill directs the World Ocean Observatory in Boothbay Harbor, Maine.
When CO2 dissolves into the sea, it starts a chemical reaction. The CO2 and water molecules combine with calcium carbonate to produce molecules called bicarbonates. And that poses a problem for all of the animals that rely on seawater’s calcium carbonate to make shells or skeletons.
Even though bicarbonate sounds like carbonate, it’s useless to sea animals, from snails and clams to sea butterflies. These animals can’t build shells from bicarbonates. And without their protective shells, such animals can die or will certainly be eaten in greater numbers. As greater amounts of carbon dioxide enter the ocean, more and more carbonates transform into bicarbonates.
But if people reduce the amount of CO2 they put in the air, then less will end up in the ocean. The United Nations’ International Panel on Climate Change is a large group of climate scientists. It has concluded that to avoid major destruction to the world’s oceans, by 2050 people must cut CO2 emissions in half.







