Explainer: What are acids and bases?
One likes to give away protons and the other likes to snatch them up
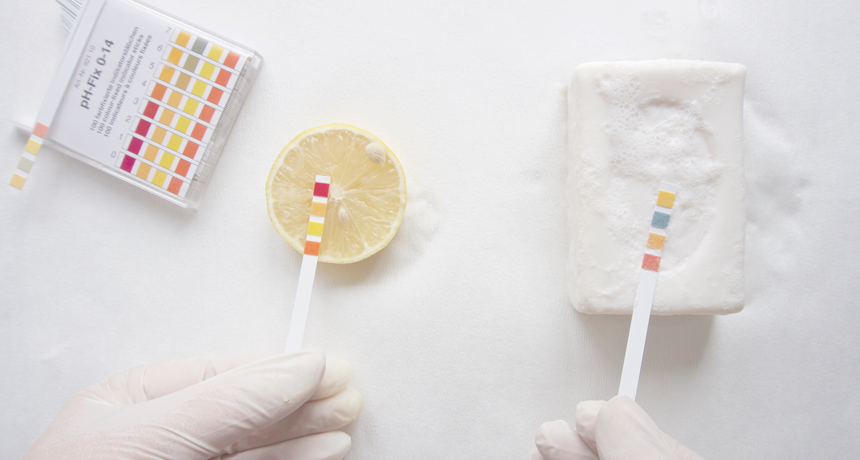
Chemists often use a test — one in which the color of a special chemical on some test strip changes — to determine if some liquid (here, lemon juice and soapy water) is an acid or a base.
ozergok/iStock/Getty Images Plus
By Lida Tunesi
If a chemist tells you soapy water is basic, she isn’t calling it simple. She’s referring to the sodium hydroxide used to make soap; it is an alkaline (AL-kuh-lin) substance. Basic — or alkaline — describes properties of certain molecules in a solution. These substances are the opposite of acids — such as the citric, ascorbic and malic acids that give lemon juice its puckering sourness.
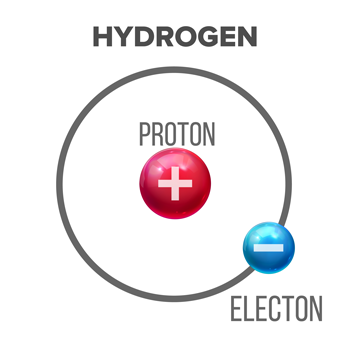
Throughout history, chemists have created different definitions of acids and bases. Today, many people use the Brønsted-Lowry version. It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. At a minimum, that tells us that all Brønsted-Lowry acids must contain hydrogen as one of their building blocks.
Hydrogen, the simplest atom, is made up of one proton and one electron. When an acid gives away its proton, it hangs on to the hydrogen atom’s electron. This is why scientists sometimes call acids proton donors. Acids will taste sour.
The type in vinegar is known as acetic (Uh-SEE-tik) acid. Its chemical formula can be written as either C2H4O2 or CH3COOH. Citric (SIT-rik) acid is what makes orange juice sour. Its chemical formula is a bit more complicated and is written as C6H8O7 or CH2COOH-C(OH)COOH-CH2COOH or C6H5O7(3−).
Brønsted-Lowry bases, in contrast, are good at stealing protons, and they’ll gladly take them from acids. One example of a base is ammonia. Its chemical formula is NH3. You can find it in many window-cleaning products.
Not to confuse you, but . . .
Scientists sometimes use another scheme — the Lewis system — to define acids and bases. Instead of protons, this Lewis definition describes what molecules do with their electrons. In fact, a Lewis acid doesn’t need to contain any hydrogen atoms at all. Lewis acids only need to be able to accept electron pairs.
Different definitions are useful for different situations, explains Jennifer Roizen. She is a chemist at Duke University in Durham, N.C. “We use both definitions in my lab,” Roizen says. “Most people use both. But a given application,” she says, “may rely on one.”
Water (H2O) is chemically neutral. That means it is neither an acid nor a base. But mix an acid with water and the water molecules will act as bases. They’ll snag hydrogen protons from the acid. The altered water molecules are now called hydronium (Hy-DROHN-ee-um).
Mix water with a base and that water will play the part of the acid. Now the water molecules give up their own protons to the base and become what are known as hydroxide (Hy-DROX-ide) molecules.
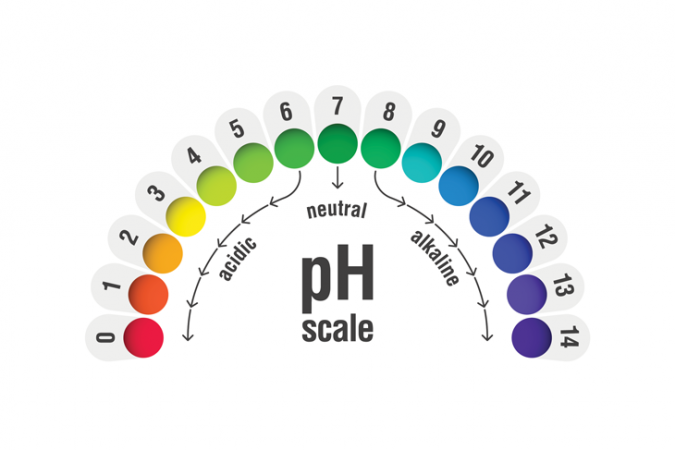
To identify acids from bases, and the relative strength of each, chemists tend to use a pH scale. Seven is neutral. Anything with a pH below 7 is acidic. Anything with a pH above 7 is basic. One of the earliest tests to determine acids from bases was the litmus test. A chemical patch turned red for acids, blue for bases. Today chemists can also use pH indicator paper, which turns every color of the rainbow to indicate how strong or weak an acid or base is.







