Explainer: What is a metal?
A weak grasp on their electrons allows these elements to conduct electricity

Molten iron, a metal, flows into a casting mold. Iron turns liquid at around 1,500º Celsius (2,750º Fahrenheit).
Jill King/EyeEm/iStock/Getty Images Plus
A towering skyscraper bends under a strong gust of wind, but it doesn’t snap. That’s one advantage metals can offer. Being malleable (MAAL-ee-ah-bul), metals can be hammered into sheets without shattering. Because they’re ductile (DUK-tul), they can be easily pulled and stretched into wires without snapping. Metals also can conduct electricity.
But not all metals are equally prized. Although more than three-fourths of the 118 elements on the periodic table are metals, we craft tools from only a few of these. That’s because familiar metals, such as iron and silver, are special. For one thing, they’re easier to find than other metals. (Although most known elements are metals, they are fairly uncommon in nature.)
Familiar metals also are less reactive than most other metals. Reactivity refers to how easily a substance reacts chemically with other substances. Low-reactivity metals are safer to handle than high-reactivity ones. Pure silver is so safe that we use it for jewelry and flatware. But pure sodium, also a metal, is so reactive it explodes on contact with water!
Good thing, then, that pure sodium never occurs naturally. Instead, we find it after it has already bonded chemically with one or more other elements. Sodium chloride, or table salt, is a common example. And that highlights another reason low-reactivity metals are so useful. They often occur naturally in forms easy to work with. For example, silver can be mined as pure silver. But if we wanted pure sodium, we’d need a way to separate it from one of the chemicals to which it had bonded. That can be hard to do.
Sometimes, a special project — such as the $10-billion James Webb Space Telescope — may call for a fairly rare metal. After 25 years in development, the telescope launched on Christmas morning in 2021. For this eye in the sky, NASA chose the rare metal beryllium (Beh-RIL-ee-um) to make its honeycomb of gold-plated mirrors. Beryllium is super lightweight. That makes it easy to launch into space. Beryllium also holds its shape in frigid temperatures. When rocketed from Earth’s relatively lukewarm air to the cryogenic (super-cold) temperatures of space, metals contract and bend. Since telescopes work by reflecting light, any tiny change in their shape could ruin a telescope’s images. But beryllium remains more stable than most metals during such sharp temperature changes.

Metal vs. nonmetal: What’s the difference?
If it’s able, an atom will steal electrons from a neighboring atom. It won’t steal all of them, but just enough to be stable. Different elements have different numbers of electrons that — at least in theory — can be stolen by a neighbor. These are called valence (VAY-lents) electrons. They are the outer, orbiting electrons that can become part of chemical bonds.
Metal atoms differ from nonmetal ones in how well they steal valence electrons from other atoms. One might say that metals are bad thieves. Instead of capturing a neighbor’s electrons, they usually give up their own. This tendency to lose electrons is described as their “metallic character.”
Nonmetallic elements, therefore, have a low metallic character. Among these nonmetals are carbon, oxygen and nitrogen. When it comes to electron thieves, nonmetals are the best. King of those nonmetals is fluorine. When it comes to electron-stealing, fluorine’s a downright bully. So it’s highly reactive.
Metals do desire electrons, but only weakly. In many ways, however, this weakness turns out to be metals’ strength. Their malleability (bendiness), ductility (stretchiness) and conductivity come from the tendency of these elements to lose electrons.
Metals can even form a special bond
Most chemical bonds occur as atoms fight over electrons. A metallic bond occurs when two metal atoms bond. Neither of them seems to care much which atom ends up with the extra electrons.
Contrast this with an ionic bond. That occurs when a metal (like sodium) bonds with a nonmetal (such as chlorine). Due to big differences in their metallic character, the nonmetal steals electrons from the metal atom and keeps those electrons.
Chemical bonding occurs after the theft. The metal and nonmetal remain stuck together because they now have opposite charges. Both atoms started with a neutral charge. Once the nonmetal gained an electron, it became negative. (That’s because electrons have a negative charge.) But the metal lost an electron. That left the metal with an overall positive charge. Notice that the metal isn’t positive because it gained a positive charge. It’s positive because it lost a negative one. These oppositely charged atoms, now called ions, attract each other and stick together.
Metal bonds also differ from the covalent bonds that form when two nonmetals join up. There, both nonmetals try to wrestle electrons from the other. But since both have strong claims on the other’s electrons, they both fail. The nonmetals end up locked in a perpetual tug-of-war. Because neither atom “wins,” these atoms are described as “sharing” their electrons.
But when two metal atoms bump into each other, they don’t fight for electrons. Neither really wants the electrons badly enough. When many metal atoms end up stuck together, as in a piece of metal, their electrons move from one atom to another to another to another. Scientists describe these electrons as “delocalized.”
Delocalized electrons explain why metals conduct electricity. After all, electricity is just the movement of electrons. One model used to explain metallic bonds envisions metal atoms as though they float through an ocean of electrons.
Delocalized electrons don’t just explain metals’ conductivity. They also explain metals’ malleability and ductility.Metallic bonds allow metal atoms to move around within their electron sea, yet still remain bonded. That would never be possible if they were locked rigidly together with covalent or ionic bonds.
The atoms in some metals move around more easily than others. Metals with easily moveable atoms are too malleable to use in tool-making. They’re too soft. Sodium metal is one such example. Sodium is malleable enough to cut with a spatula. Most of our really useful metals, especially those used for making tools, are hard enough to keep their shape. Take iron, for instance. To forge an iron sword, a blacksmith must reposition bajillions of iron atoms. And that’s no easy task.
So, then, how do blacksmiths do it? Keep in mind that atoms never sit still. They move and shake. And hot atoms shake more than cold ones, giving hot atoms more freedom. In molten metal, the bonds between atoms weaken a lot. So a blacksmith will boost a forge’s heat to upwards of 1,600º Celsius (2,900º Fahrenheit). This intense fever weakens those metallic bonds, allowing the blacksmith to hammer the metal into shape.
Once the new sword cools, its atoms slow and the metallic bonds re-secure.
Alloys and a truly Titanic lesson
Jewelry almost never contains pure gold. It would simply be too soft. So metal-workers mix gold with other metals, often copper, to make it less malleable. Such mixed metals are called alloys. Alloys can also be metals mixed with a tiny dash of some nonmetal.
Carbon steel is an iron alloy made with a pinch of carbon. Skyscrapers and bridges are made from lots of carbon steel. So are many kitchen knives and screwdrivers. The carbon in carbon steel reinforces the iron, making it harder. But increasing a metal’s hardness also drops its malleability.
In fact, too much carbon will make steel brittle. And as metals get cold, this problem only worsens. In April 1912, the cruise ship Titanic struck an iceberg on its maiden voyage and sank. More than 1,500 people died. Studies on the ship’s gashed hull suggest that the Titanic‘s steel became brittle in the Atlantic’s frigid waters. The Titanic’s steel alloy became brittle at 32 ºC (90 ºF). The night Titanic sank, the water was just –2 ºC (28 ºF).
Engineers today would use a different steel. For example, a modern steel-type called ASTM A36 can handle –27 ºC (–17 ºF) before becoming as brittle as the Titanic‘s had. The difference is in the alloy. The Titanic‘s steel “had a lot more sulfur content and a lot less manganese,” explains Andrew Falkowski. He’s a materials engineer in Salt Lake City, Utah. He also co-hosts the Materialism Podcast, a show about materials science.
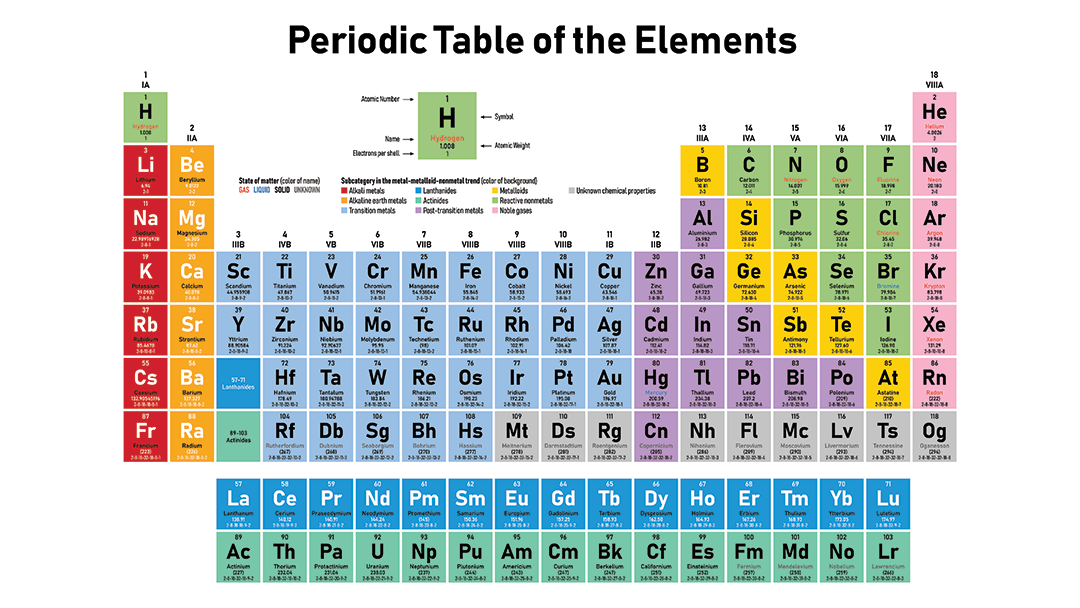
The periodic table separates metals from nonmetals
You can tell how metallic any element is just by finding it on the periodic table. Metallic character exists on a spectrum. Elements fall somewhere between the least metallic — fluorine — and the most metallic — cesium (or francium, if you include lab-made elements). So in terms of metallic character, cesium and fluorine are polar opposites. Fluorine is the most reactive nonmetal in existence. And cesium is the most reactive metal. If these two ever meet, they’ll burst into intense white fire.
Find these elements on a periodic table, and you’ll notice something. They’re on opposite sides. That’s no coincidence.
Nonmetals occupy the upper right of the classic periodic table, including the entire far-right column. There’s one exception. Hydrogen is the only nonmetal that is not grouped with the nonmetals. Hydrogen is weird, mainly because its atoms are so tiny.
Metals occupy everywhere except the upper right. As you move away from the nonmetals, metallic character increases. It increases as you move from right to left and also as you move down.
Similar types of metals group together on the periodic table. For example, the far-left column contains sodium and the other so-called alkali metals. All of these react violently with nonmetals. The elements that lie between metals and nonmetals are called metalloids or semi-metals. They have properties of both metals and nonmetals. Arsenic and silicon are examples. In the middle are what are known as transition metals. That’s where most familiar metals live, such as gold, silver and copper.







