Explainer: What is an electrode?
Simplifying the sometimes-confusing terms that describe where electricity meets chemistry

All batteries have a negatively charged anode at one end and a positively charged cathode at the other end. These are their electrodes.
Peter Dazeley/The Image Bank/Getty Images
Electricity is essential to life and much of what happens throughout the cosmos. But this flow of electrons does not just occur anywhere and everywhere. It flows as a current and along paths. Metals offer a great path. That’s why scientists refer to metals as conductors: They conduct electricity. But to move electricity through non-metallic materials, scientists need an electrolyte. This is a substance that contains ions — charged particles — that allow the current to flow.
To make this flow happen, chemists place electrodes in contact with the electrolyte.
Electrodes often are made of metal. Sometimes they are graphite. But whatever they’re made of, electrodes must be conductors. These electrodes are key parts of what scientists call an electrochemical cell.
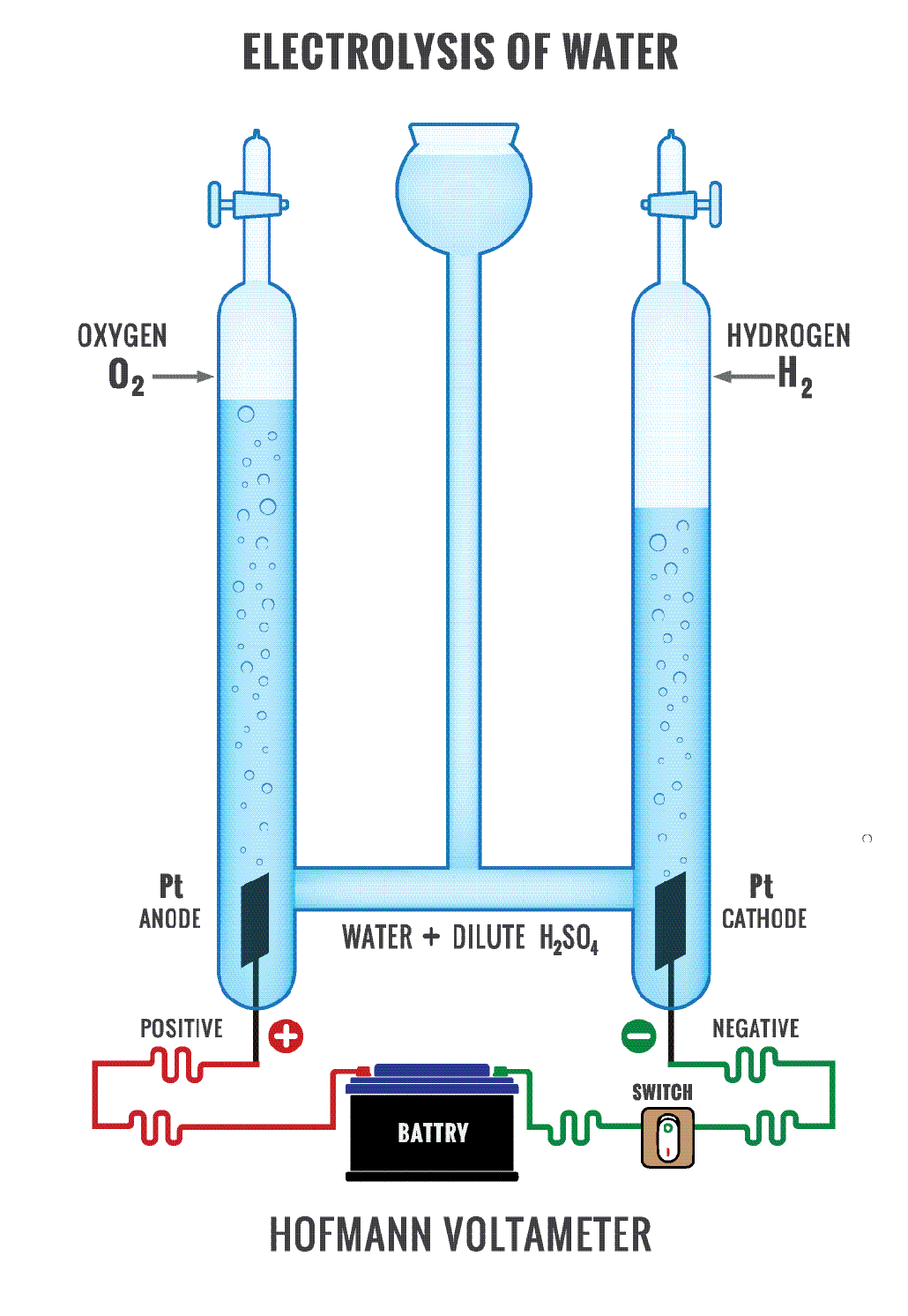
Such cells do either of two things. One type generates electricity from chemical reactions that occur naturally. This is a battery. Chemists refer to these reactions as spontaneous. The second type of cell uses electricity to force otherwise unwilling chemical reactions to occur. This is known as an electrolysis cell. Its reactions are non-spontaneous. A common example of electrolysis is the use of energy to split water into hydrogen and oxygen.
Each cell has two electrodes. They’re the positively and negatively charged halves of the system. One electrode is the anode (AN-oad). The other is the cathode (KATH-oad). And here’s where the confusion can set in. Depending on the type of cell, the positive and the negative electrodes can carry opposite names.
The role of spontaneity
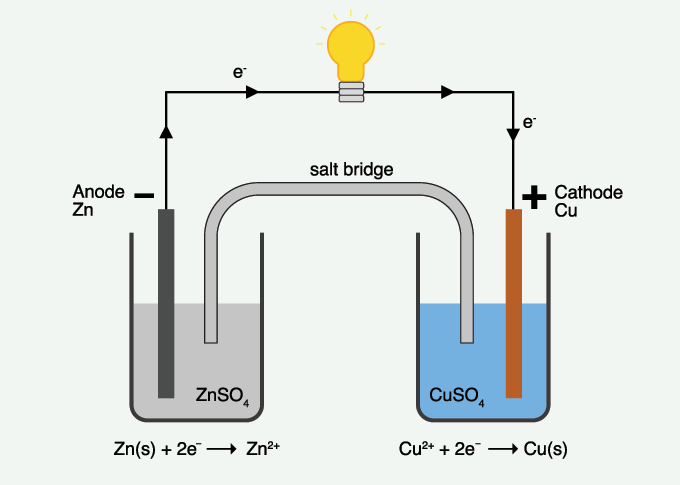
The chemical reactions in a battery generate a flow of electrons through a process where one chemical loses electrons to another chemical. A wire connects the two reacting substances. The flow of electrons through that wire is electricity.
The battery’s negative electrode is the anode. Its positive electrode is the cathode. Since electrons are negatively charged, they naturally flow from the negative anode toward the positive cathode. The process of losing electrons is known as oxidation. In a battery, this takes place at the anode. Reduction is the process of gaining electrons. It takes place at the cathode. In a copper-and-zinc battery, for instance, zinc loses electrons to the copper.
In an electrolysis cell, electricity triggers a non-spontaneous reaction. Here, too, one chemical loses electrons as a second gains them. But it’s the opposite of a battery.
The cathode now is the negative electrode. The anode is the positive electrode.
Electrons still flow toward the cathode. Because it’s electrolysis, this system forces electrons to do something that they don’t do naturally. They flow in the opposite direction they would naturally choose. So the splitting of water into hydrogen and oxygen in an electrolysis cell causes the process of losing electrons (or oxidation) to take place at the anode. Gaining electrons (or reduction) takes place at the cathode.
How can you remember this? No matter what type of cell, reduction always takes place at the cathode. So electrons always flow toward that cathode. And that should help you keep the names for these electrodes straight.