Explainer: What the pH scale tells us
We think of it as distinguishing acids from bases, but it’s really about proton donors and snatchers

Indicator paper is an easy way to measure pH. It changes color depending on how acidic or basic a mixture is.
iprogressman/iStock/Getty Images Plus
By Lida Tunesi
The white vinegar in your kitchen cupboard has a pH of about 2.4. The pH of oven cleaner is around 13. What do these numbers mean? They give us a clue to what types of molecules are in these hydrogen-containing solutions — acids or bases — and how they will interact with the molecules around them.
One system that scientists use to define acids and bases is called the Brønsted-Lowry theory. (It’s named after two scientists who proposed it.) The Brønsted-Lowry definition says that an acid is a molecule that will give away a proton from one of its hydrogen atoms. A proton is a positively charged particle (and is the nucleus of the hydrogen atom). On a pH scale, acids all fall below 7.
The opposite of an acid is a base. Chemists describe these molecules as being alkaline (AL-kuh-lin). Brønsted-Lowry bases are good at stealing protons and will gladly take them from acids. One example of a base is ammonia. Its chemical formula is NH3. You can find ammonia in window-cleaning products. Bases all come in above 7 on the pH scale.
The role of hydrogen gives rise to the term pH. That term arose around 1909 from the German for potenz (meaning power) and hydrogen (whose chemical symbol is a capital H). So it’s a measure of a solution’s willingness to give or take a hydrogen’s proton.
However, chemists also talk about Lewis acids and Lewis bases. In the Lewis theory, acids and bases don’t necessarily contain any hydrogen atoms. They are labeled acids or bases depending on whether they donate or accept pairs of electrons.
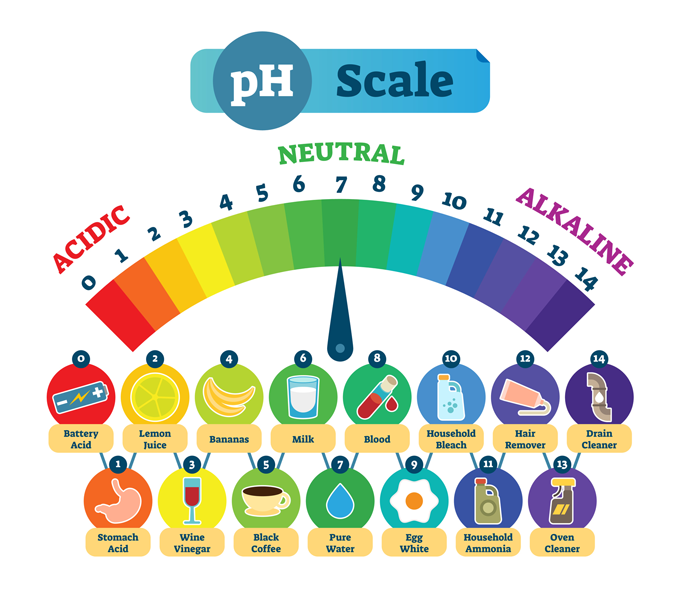
Most images show the pH scale going from zero to 14. This scale is logarithmic, so there is a 10-fold difference in strength between each number.
Pure water is neutral, neither an acid nor base. As such, it sits smack in the middle of the pH scale at 7. But mix an acid with water and the water molecules will act as bases. They’ll snag hydrogen protons from the acid. The altered water molecules are now called hydronium (Hy-DROHN-ee-um).
Mix water with a base and that water will play the part of the acid. Now the water molecules give up their own protons to the base and become what are known as hydroxide (Hy-DROX-ide) molecules.
The pH scale measures whether there is more hydronium or hydroxide in a solution. In other words, it tells us how basic or acidic the solution is. A lower pH means something is more acidic, also known as a stronger acid. A higher pH means it is more alkaline or a stronger base.
Chemistry classes will often use a litmus test to identify acids from bases. A blue litmus paper turns red in acids while a red litmus paper turns blue in basic solutions. Other pH indicator papers are available that will actually identify the rough pH of some acid or base, also using color-change chemicals.







