A new drug mix helps frogs regrow amputated legs
Coaxing animals to regrow limbs could be a stepping stone to giving people the same ability

Adult African clawed frogs can’t fully regrow lost body parts on their own. But a new device that holds a chemical cocktail at the wound site has coaxed some frogs to regrow useful limbs.
Brian Gratwicke/Flickr (CC BY 2.0)
Share this:
- Share via email (Opens in new window) Email
- Click to share on Facebook (Opens in new window) Facebook
- Click to share on X (Opens in new window) X
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Reddit (Opens in new window) Reddit
- Share to Google Classroom (Opens in new window) Google Classroom
- Click to print (Opens in new window) Print
Some animals have a fantastic ability to regrow limbs. Salamanders sprout new tails. Starfish develop new arms. Some sea slugs can even rebuild their entire bodies from the head down. Humans, unfortunately, do not boast this talent. But a new study in frogs offers hope that technology might someday grant people a similar power of regeneration.
Researchers started by building a device to help frogs regrow missing legs. This device delivers a mix of drugs to the site where a frog’s leg had been amputated. Adult frogs, like humans, cannot generally regrow body parts on their own. But wounds in frogs treated with the new device could.
Researchers describe how they did it in the January 26 Science Advances.
Coaxing a frog’s body to make a new leg from scratch isn’t as wild as it may seem, says Michael Levin. He’s a developmental biologist at Tufts University in Medford, Mass. “The cells of the frog already know how to make frog legs,” he says. After all, a frog’s cells already grew legs when the animal was a developing embryo. “Our goal is to figure out how to convince them to do it again.”

BioDome benefits
Levin’s team amputated the right back leg of 115 adult African clawed frogs (Xenopus laevis). These legs were cut off at the knee. The frog amputees were then split into three groups.
Researchers covered wounds in the first group with silicone sleeves. Called “BioDomes,” these held a silk-based gel. The second group were treated with BioDomes that held a gel containing five drugs. They included a growth hormone, a nerve growth promoter and a substance to reduce inflammation. The two groups of frogs that got BioDomes kept the sleeves on for 24 hours only. Frogs in the third group received no treatment.
After about four months, something curious happened to frogs that had gotten BioDomes with the drug cocktail. “We started to see a slight difference in the leg shape,” says Nirosha Murugan. She’s a cancer biologist now at Algoma University in Sault Ste. Marie, Canada. “With time, that bud … started to take shape into a whole leg.”
After 18 months, those frogs had regrown their missing limbs. The new limbs even had nubs where toes would typically be. The amputees kicked, stood and pushed off the walls of their tanks using these new legs.
Frogs that got BioDomes without any chemicals didn’t do quite as well. The BioDome itself did promote some regeneration. The sleeve created stiffness and pressure at the wound site that seemed to spur growth, Murugan says. But frogs that got BioDome with drugs grew longer legs with thicker bones. They also had more blood vessels and nerves in the new limbs. Plus, their regrown limbs were more sensitive to touch.
“It’s actually remarkable that just a single treatment on one day can cause all this change,” she says. Frogs that didn’t get any treatment, meanwhile, grew spiky flaps at the wound site. These were basically stumps with no function.
Lost legs
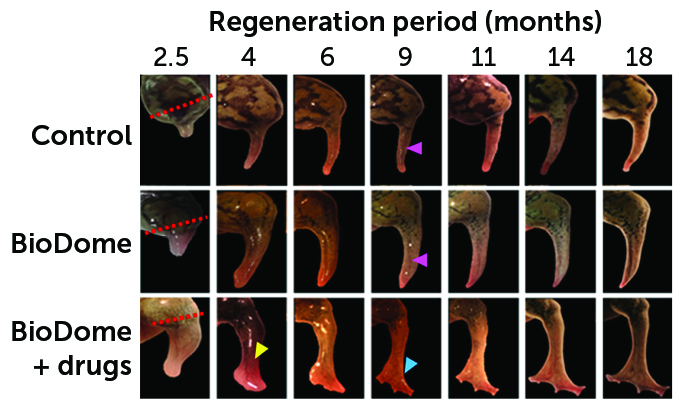
During 18 months following an amputation, frogs grew new limbs. (The site of each amputation is marked with dashed lines in the left column.) One-third of the frogs were in a “control” group and didn’t receive any treatment. Those frogs grew a spiky stump at the site of their amputation (one shown in the top row). Another third of the frogs received BioDome devices that covered their injury for 24 hours (middle row). The remaining frogs received the BioDome and a drug cocktail (bottom row). The last group grew the longest limbs with the biggest bones. Their limbs also had more blood vessels and nerves. And they developed legs with a paddle-like shape (yellow arrow) with toelike buds (blue arrow). The other groups, meanwhile, grew only spiky flaps (pink arrows).
Saving lives and limbs
This first attempt at using a chemical cocktail to coax limb regrowth is “a great start,” says John Barker. He’s an orthopedic researcher who did not take part in the new study. He recently retired from Goethe University Frankfurt in Germany. By tweaking the drug cocktail, he says, “there’s no end to what you could try.”
Levin’s team has moved on to similar work in mice. These experiments use the same cocktail from the frog study along with new ones. Levin’s past research had shown electricity plays a role in shaping the growth of body parts. So he and his colleagues are adding compounds to the cocktail that alter the electrical state of cells.
Someday, scientists want to regrow human limbs and organs. Like frogs, human bodies know how to remake parts, Barker says. Kids younger than about 10, for example, can regrow lost fingertips. In health care, the possibility of “regeneration changes everything,” Barker adds. “Instead of treating symptoms, you could literally cure a disease.” Imagine regrowing heart tissue to replace damaged parts of the organ and improve heart function.
That, however, is a long way off. And mending hearts might actually be easier than remaking limbs. Several types of tissue must work together to build a limb. And there’s still much that researchers don’t know about how bodies form their parts.
“We don’t understand how collections of cells solve problems,” Levin says. How do cells decide what to build or even when to stop? Regenerating lost tissues “is going to require us to do much better about understanding that.”







