Sickle-cell gene therapies offer hope — and challenges
Doctor Erica Esrick discusses existing treatments and an ongoing clinical trial
People who make these crescent — or sickle-shaped — red blood cells face a lifetime of pain and risk of disease. But gene therapy promises to help the body make healthy, roundish blood cells again.
Stocktrek Images/Getty Images Plus
Medicines used to consist only of potions, salves and pills that doctors dispensed to treat or cure disease. Most have come a long way since your great-grandparents’ day. In fact, scientists are learning to treat some of the most troubling ailments — inherited diseases — at the cellular level. These therapies attack the problem at its source: our genes. One such therapy that shows particular promise takes aim at the first known inherited genetic disorder: sickle cell disease.
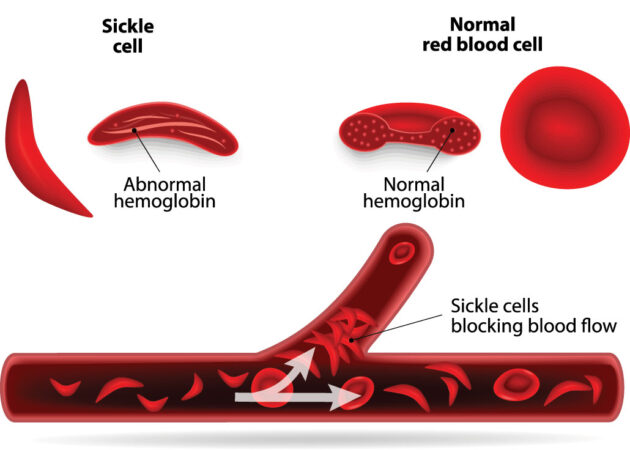
The rate of people born with this disease varies around the globe. In the United States, about 3 in every 200 babies are born with the mutation that causes their red blood cells to sickle (take a curved shape). These cells don’t live long. Since they carry oxygen throughout the body, their untimely demise causes anemia. It also decreases the delivery of oxygen to cells throughout the body. Their unusual sickle shape also means affected blood cells can block blood vessels, causing intense pain and worse, such as a stroke.
Gene therapies look to replace, turn off or fix missing or broken genes. Some could bring a true cure. But the journey to today’s handful of approved gene therapies — including certain blood cancers — has been rocky. Human trials in the 1990s didn’t achieve success. Others a decade later brought unintended and sometimes deadly impacts, including leukemia.
But many researchers believe sickle cell is an especially good target for gene therapy. The reason: Its underlying genetic problem is well understood.
Erica Esrick is co-leading a clinical trial that is testing a gene therapy for sickle cell disease. She’s a pediatrician at Boston Children’s Hospital and Harvard Medical School. Both are in Boston, Mass. The new treatment works to encourage the body to make more of a healthy type of hemoglobin.
Hemoglobin is the molecule in red blood cells that carries oxygen throughout the body. Malfunctioning hemoglobin causes sickle cell disease. The healthy type of hemoglobin that Esrick’s treatment encourages the body to produce is known as fetal hemoglobin. It’s the type made by infants and young babies, but not adults.

The therapy uses harmless parts of a virus to deliver a snippet of DNA into cells. When used this way, the virus is a vector or carrier.
That vector delivers DNA — a type of genetic material — to the patient’s own bone marrow cells. That DNA contains the instructions to make a short string of genetic material, called a microRNA. This RNA, Esrick explains, provides new genetic instructions telling those cells to make more fetal hemoglobin.
Her team’s therapy halts production of a protein that normally keeps fetal hemoglobin from being made in anyone who isn’t a baby. Like turning on a faucet, this change means red blood cells can now deliver a steady stream of oxygen-carrying fetal hemoglobin.
Preliminary data show this treatment helped six sickle cell patients make fetal hemoglobin. Esrick and her colleagues described this in January 2021 in the New England Journal of Medicine.
The patients’ symptoms were reduced or went away throughout a follow-up period, which lasted from several months to more than two years. The team has now expanded this trial to include more patients.
Tests of other gene treatments to tackle sickle cell are underway elsewhere. One biotechnology company called bluebird bio is testing an approach that gives patients a normal copy of the hemoglobin gene (to replace their mutant gene). Another team is preparing to begin a trial that will edit that gene directly. It will use CRISPR technology.
Science News staff writer Erin Garcia de Jesús spoke with Esrick about her team’s ongoing trial. Esrick’s answers have been edited here for length and clarity.
What tools do we currently have to treat sickle cell?
The only cure is a bone marrow transplant. The bone marrow is like your body’s blood cell factory. If you can get bone marrow from somebody who doesn’t have sickle cell disease, then you can grow your own healthy red blood cells that don’t sickle.
But getting a transplant is a big deal. And it’s really only standard if you have what’s called a matched sibling. That’s a brother or sister without sickle cell who has key white-blood-cell proteins that match yours.
Fewer than one in every five people with sickle cell disease have such a matched sibling available. Where one is, this becomes a good potential treatment. But it still has risks. There is a small chance a transplant patient may die. There are also a lot of potential side effects. Among them is a chance the body will try to reject the new cells. Or the new cells may react against the body. To limit those risks, people must take drugs to suppress their immune system. But these drugs put patients at a higher risk of infection.
There are also medications to treat sickle cell. The most well-established is called hydroxyurea (Hy-DROX-ee-yu-REE-uh). It increases fetal hemoglobin. In many people, it increases fetal hemoglobin by a lot. That’s why it works so well. It’s been available since the ’90s, and has been moving gradually to use in younger and younger patients.
Today, there is a very clear recommendation that essentially every child with sickle cell should be on it. But not everyone has access to specialists who prescribe this medication. It also has to be taken daily. And there are downsides. Some people have side effects so bad they can’t take it. And the drug doesn’t work in everybody.
How many people are in your team’s trial and what results have you seen so far?
Nine patients have been treated. We anticipate the tenth patient will be treated soon. Early data from the first six patients were published [see above]. Additional data from subsequent patients have been largely quite similar — except for one patient (whose fetal hemoglobin response was not as strong). In a trial this small, it is still too early to draw long-term conclusions.
What is the process like for trial participants?
Patients have to get their cells collected. These are the cells that live in the bone marrow and give rise to blood cells. Getting them takes a three-day hospital stay. Sometimes this collection step has to be repeated a few times. The cells are removed by IV, basically, and then are sent to the lab.
When we get word from the lab that “OK, we have a good product” — meaning the virus got the DNA into enough cells — the patient will come back to the hospital. And they’ll need to stay for a month or so. Because they’ll need to receive chemotherapy, it’s a long and uncomfortable stay.
That chemotherapy is needed to nearly wipe out the old bone marrow cells that weren’t collected and are still in the body. Eliminating them will give less competition to those cells that are given back to the body. This improves their chances to set up shop and expand.
Chemotherapy comes with a lot of side effects and risks. Short-term ones can include temporary hair loss, nausea and pain with swallowing. Chemotherapy brings longer-term risks. These can include infertility and a risk of blood cancers.
Why choose gene therapy over a bone marrow transplant if both require chemotherapy?
With gene therapy, you’re getting back your own cells. So there’s no issue with the immune system trying to reject them. To avoid this risk in people who get a transplant from another person, patients have to be on immunosuppressive medications for months. That is not necessary with gene therapy.
The other risk in a bone marrow transplant from another person is graft-versus-host disease. Here the transplanted tissue and donated cells reject the recipient. That can cause severe disease. With gene therapy, that’s not a risk at all.
Last year, the clinical trial run by bluebird bio announced that one trial participant developed leukemia. Cancer is obviously a huge concern and has thwarted previous gene-therapy trials. What do we know so far about that?
This was, of course, of major concern to the field. It was actually the second case of leukemia in that trial. The first one was published a couple of years ago.
If there’s ever a case of leukemia or any pre-leukemia in a gene-therapy trial, we always ask: Was it caused because the vector stuck a gene into a spot that was dangerous?
It does not look like that was the case here. The first patient in the bluebird bio trial who developed leukemia didn’t have the transferred gene in those leukemia cells. So, the thought was that this was probably a case of chemotherapy causing leukemia. We do know that can be a risk for a very small share of people who get chemotherapy.
But the second case, in February 2021, raised a red flag. Why did that happen two times in a trial of only 40-something patients? It’s still not clear. Some studies have suggested that people with sickle cell disease may have an increased risk of leukemia. While some investigations were done, the U.S. Food and Drug Administration placed that trial on hold. When evidence suggested that this leukemia wasn’t directly related to the vector, FDA allowed the trial to reopen.
Our trial, which has many similarities to the bluebird bio trial, was not put on hold by the FDA. But it was put on hold by our funder, the National Heart, Lung and Blood Institute. After they looked at the data, that hold was recently lifted.
Have there been any cases of leukemia in your team’s trial?
Fortunately, no.
What are some of the biggest challenges that sickle cell has had to overcome?
For the longest time, there were no new therapies at all. These technologies took a long time because they are based on basic science discoveries that were being worked on in labs. But also, the patient population with sickle cell is a population that has historically been underserved and without a lot of power.
In the United States, it’s primarily Black and Latino patients, and those populations have suffered from health inequality. Sadly, I think that if there were a disease that caused this degree of illness, death and pain in other parts of the population, it may have been speedier.
What gives you hope? What do you find exciting?
We have to be careful to avoid sending a message like “We have a cure!” After all, the trials are still small and early. But that said, it is really exciting that because there’s no need to find a bone marrow match, this is a treatment that is theoretically possible for everyone. That’s a huge difference from classic bone marrow transplants.
The speed at which gene therapy treatments are being developed is amazing. I think the horizon is very bright in terms of one or hopefully many of these therapies being really effective and safe.
I’ve talked to so many patients and families who have reached out interested in our trial or other trials. There’s such a huge unmet need.
UPDATE: On December 8, 2023, the U.S. Food and Drug Administration approved the first gene-editing therapy for sickle-cell disease in people 12 years and older. It could offer relief for people with severe forms of the painful disease. Known as Casgevy, the new treatment is the world’s first to tweak cellular genes through use of the molecular scissors known as CRISPR/Cas9.







