By not including everyone, genome science has blind spots
Too little diversity in genetic databases is making precision medicine difficult for many

A lack of diversity in genetic databases is making precision medicine less effective for many people.
Delphine Lee
It’s been about 20 years since scientists unveiled a rough draft of the human genome. This is all of the DNA found in a human cell. Think of it like a genetic instruction book for the body. Creating that rough draft was like a medical moonshot. It held out the promise that doctors might soon be able look at someone’s DNA and prescribe the right medicines for their illness. They might even prevent certain diseases.
That promise is known as precision medicine. But it has yet to be fulfilled in any widespread way.
Researchers are getting clues about some DNA variants linked to certain conditions. Some people have them. Others might not. And scientists have figured out how some variants affect the way drugs work in the body. But many of those advances have helped just one group: people whose ancestors came from Europe. In other words, white people.
What’s generally known as the human genome does not represent everyone, says Constance Hilliard. “What we have is essentially a European genome.” Hilliard is an evolutionary historian. She works at the University of North Texas in Denton. Unless all your ancestors are from Europe, she says, that human genome can’t tell your whole story.
Much of the work on precision medicine relies on genome-wide association studies. They’re known as GWAS. These are mostly based on data from white people. But skin color isn’t the issue. The problem is that those data don’t represent the full range of human genetic diversity.
A person may go to the doctor to have their DNA tested. They may want to know if they inherited a variant that may cause cancer. Or they may want to know if a certain drug will work for them. Especially if that person is not white, they’re often left with more questions than answers. There aren’t enough data yet to make useful conclusions. This is true for African-American people. It also applies to people of Asian, Native American and Pacific Island ancestry. But that situation could change if genetics included a more diverse group of people, researchers agree.
Hilliard’s solution is to make customized reference genomes for populations that face worse health outcomes. The more specific the better.
For instance, many African Americans are descended from enslaved people. They have origins and histories distinct from those of recent African immigrants. Those unique histories may leave stamps in their DNA. And that can make a difference in their health today. The same goes for Indigenous people from various parts of the world. It’s also true for Latino people from Mexico versus the Caribbean or Central or South America.
Researchers have made efforts to boost diversity in genetic studies. That includes geographic, social and economic diversity. However, there is still a long way to go. How to involve more people of diverse backgrounds in genetic research raises thorny questions of ethics. These include how to treat all people fairly and equitably.
Science News magazine sought to bring readers into this conversation. It posed some questions for readers. Each had watched a short video of Hilliard explaining her views.
Respondents to this unscientific survey said that genetic research is important for improving medical care. But there were mixed feelings among the mostly white respondents about how to expand this to people of many backgrounds. Many were concerned that pointing out genetic differences might reinforce old concepts of racial inferiority and superiority.
When origins matter
Hilliard has a hypothesis: Precision medicine is more likely to succeed when researchers consider the histories of groups who face different risks for certain health impacts. U.S. Black populations are one such group. They have higher rates of kidney disease and high blood pressure. They also are more likely to die of certain cancers than other ethnic groups.
An example of why this is important comes from Hilliard’s work. Many African Americans descend from enslaved people. They die at higher rates than whites from certain types of breast and prostate cancers. They also have lower rates of a brittle bone disease. It’s known as osteoporosis (Os-tee-oh-por-OH-sis). African Americans have a variant of a gene that helps their cells take up calcium. That finding may help explain the low rates of osteoporosis in Black women.
Here’s how it might work. The variant, known as the TRPV6, traces back to the ancestors of some African Americans. These are Niger-Congo speakers in West Africa. In that part of Africa, the tsetse fly kills cattle. This makes it hard to do dairy farming. Without access to dairy products, people typically consumed about 200 to 400 milligrams of calcium per day. Hilliard’s hypothesis suggests that the calcium-absorbing version of TRPV6 helps their bodies meet their calcium needs.
Doctors may assume that African-American women have the same calcium needs as women of European descent. That would lead them to recommend that these women up their calcium intake. But there is a problem. The version of TRPV6 that takes in more calcium may cause some cancers to grow quickly. So eating more calcium may speed the growth of breast and prostate cancers. Hilliard reported this in the Journal of Cancer Research and Therapy in 2018.
“Nobody is connecting the dots,” Hilliard says. That may be because most research has focused on the European version of TRPV6.

Why is genetics so white?
Some Science News readers asked why genetic research became so white. And they wanted to know, what we can do about it?
Let’s start with the Human Genome Project. It created the human reference genome. That’s a sort of master blueprint of the human genetic makeup.
The reference genome isn’t one person’s genome. It is a mishmash of more than 60 people’s DNA. Many think the reference genome is mostly white. But it’s not, points out Valerie Schneider. She’s a staff scientist at the National Library of Medicine in Bethesda, Md. She’s also a member of the Genome Reference Consortium. This is the group charged with maintaining the reference genome.
About 70 percent of the DNA in the reference came from one man. About half of his DNA was inherited from African ancestors. The other half came from European ancestors. Another 10 people together donated 23 percent of the reference’s DNA. One of those people was East Asian. And 50 more people collectively provided the remaining 7 percent of DNA. Their heritage isn’t known.
Humans all have basically the same DNA. Any two people are 99.9 percent genetically identical. That’s why having a reference genome makes sense. But there’s a 0.1 percent difference between people. This reflects all the spelling variations, typos, insertions and deletions sprinkled through the human instruction book. Those differences contribute to health and diseases.
Much of what is known about how those differences affect health comes from GWAS research. In such studies, scientists start with DNA from people with certain diseases. Then they compare it to DNA from people without the disease. The aim is to find common variants that might explain why one person might get that illness while another does not.
As of 2019, 78.4 percent of people who participated in GWAS had European ancestors. One reason volunteers for the studies are mostly white, says Sam Oh, is that the majority of research funded by the U.S. National Institutes of Health is done by scientists who identify as white. Oh is an epidemiologist. He works at the University of California, San Francisco. Black and Hispanic researchers combined get only about 6 percent of research grants.
Change is slow
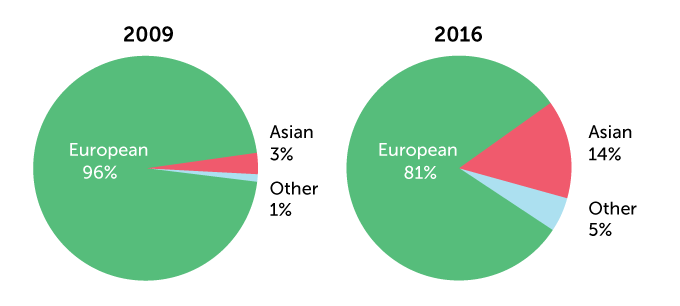
Much of the DNA in genetic databases that are used to develop precision medicine comes mainly from people of European ancestry. A comparison of 2009 with 2016 shows that there has been a slight improvement. By 2019, European ancestry had dropped to 78.4 percent of the DNA.
Ancestry of individuals in genome-wide association studies
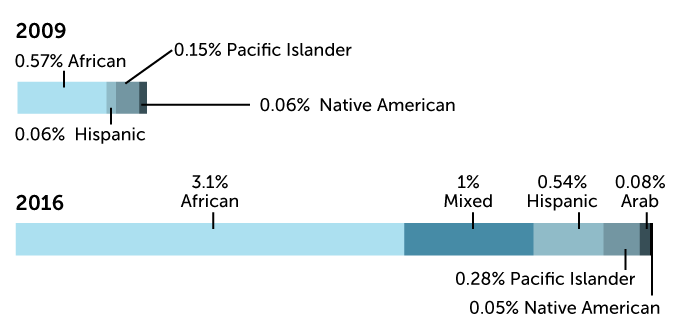
Zooming in on “other” from the data above
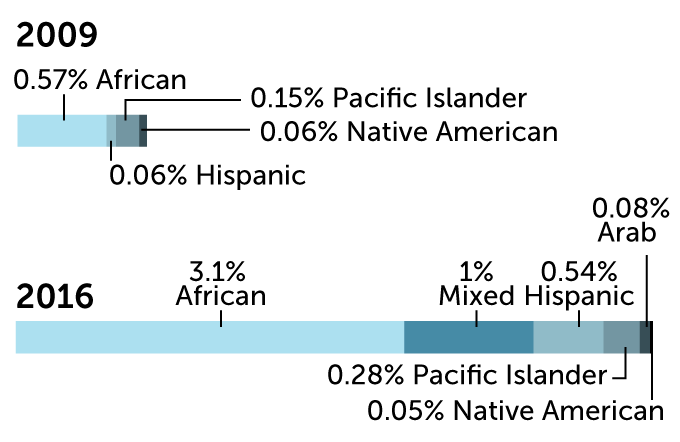
“Generally, the participants who are easier to recruit are people who look like the scientists themselves.” When people share a similar language or culture, he says, “it’s easier to establish a rapport. And you may already have inroads into communities you’re trying to recruit.”

What is diversity in genetics?
Getting people from all over the world to take part in genetic research might seem the way to boost diversity. But that won’t work, Hilliard says. If you really want genetic diversity, she argues, look to Africa.
Humans originated in Africa. The continent is home to the most genetically diverse people in the world. Ancestors of Europeans, Asians, Native Americans and Pacific Islanders carried only part of that diversity when they left Africa. So sequencing their genomes won’t capture the full range of variants, she contends.
That’s why she recommends separate reference genomes or similar tools be created for distinct groups of people. Those tools could help with health problems that may be linked to their particular genetic ancestry.
Some countries already have launched such efforts. China compiled a reference genome for the Han Chinese. It’s the world’s largest ethnic group. And a recent analysis indicates this group can be divided into six subgroups. They hail from different parts of China. This genome project also is compiling data on nine ethnic minorities in China. Denmark, Japan and South Korea also are creating country-specific reference genomes. And they’re cataloging genetic variants that might contribute to health problems their populations face.
Whether this approach will actually improve medical care remains unknown.
People often have the notion that human groups exist as discrete, isolated populations, says Alice Popejoy. “But we really have, as a human species, been moving around and mixing and mingling for hundreds of thousands of years,” she says. A public health geneticist and computational biologist, Popejoy works at Stanford University in California.
“It gets very complicated,” she says, “when you start talking about different reference genomes for different groups.” There are no easy dividing lines. Even if separate reference genomes were built, it’s not clear how a doctor would decide which would apply to a patient.

Increased discrimination?
One big drawback to Hilliard’s proposal might be social, not scientific. Many people who took the Science News survey expressed a similar concern. They worry that even well-meaning scientists might do research that somehow increases bias and discrimination toward certain groups. As one reader put it, “The fear is that any differences that are found would be exploited by those who want to denigrate others.”
Indeed, China has come under fire for using DNA to identify members of the Uighur (WE-gur) Muslim ethnic group. Its people have been singled out for surveillance by the Chinese government. It’s even sent some Uighurs to “re-education camps.”
People need a better understanding of what it means when geneticists talk about human diversity, says Charles Rotimi. He’s a geneticist at the National Human Genome Research Institute, or NHGRI. It’s in Bethesda, Md. All people are basically the same, genetically. But the human genome is able to adapt to different environments. That’s why people can carry signatures of some of the places in which their ancestors settled. “We need to understand,” he says, “how this influenced our biology and our history.”
And modern scientists, even though they weren’t involved, need to acknowledge discrimination in the past, says Lawrence Brody. He is director Genomics and Society at NHGRI. One example is the infamous Tuskegee experiment. African-American men with syphilis were not given treatment that could have cured the infection.
“We want the fruits of genetic research to be shared by everyone,” Brody says. “The ethical issue is to make sure you do it.”

Different priorities
Certain minority groups are steering clear of genetic studies, however. They are doing this even as scientists try to recruit them for such research. Promising that communities that donate their DNA will someday reap the benefits can be a hard sell.
“We’re telling these communities that this is going to reduce health disparities,” says Keolu Fox. This human geneticist is a Native Hawaiian. He works at the University of California, San Diego. So far, he says, precision medicine has not helped reduce complex diseases. That’s especially true in communities of color, he says.
That’s because “we have a real basic infrastructure problem in this country. Sixty million people don’t have health care. We have people on [Native American] reservations that don’t have access to clean water.” Some don’t have Internet. Improving infrastructure and access to health care is needed. This would do much more to erase health disparities, he says, than any genetics project could right now.
Krystal Tsosie is member of the Navajo (Diné) Nation. A geneticist, she works at Vanderbilt University in Nashville, Tenn. Tsosie also is cofounder of the Native Biodata Consortium. “Many people ask, ‘How do we get Indigenous people comfortable with engaging with genomics?’” Instead, she says, researchers should be asking how to protect tribes if they choose to engage in genetic research.
What would you like to tell the scientists working in this area? Send your thoughts to feedback@sciencenews.org.
And issues of privacy become a big deal for small groups. The 574 recognized Native American tribal nations in the United States are such small groups. So are isolated religious or cultural groups, such as the Amish. If one member of a group decides to give their DNA to a genetics project, their identity may be revealed. And that submission may paint a genetic portrait of every member of their small group. Such decisions shouldn’t be left in the hands of individuals, Tsosie argues. It should be a community decision.
Hilliard thinks people’s resistance to taking part in genetic research is about more than the fear of being singled out. It’s the result of being experimented on and then seeing medical breakthroughs from those studies benefit only whites.
“Medical researchers just need to accomplish something that benefits somebody other than Europeans,” she says.
This project on ethics and science was supported by the Kavli Foundation.







