How to make a ‘three-parent’ baby
Scientists combined an egg, sperm and some donor DNA to create a baby
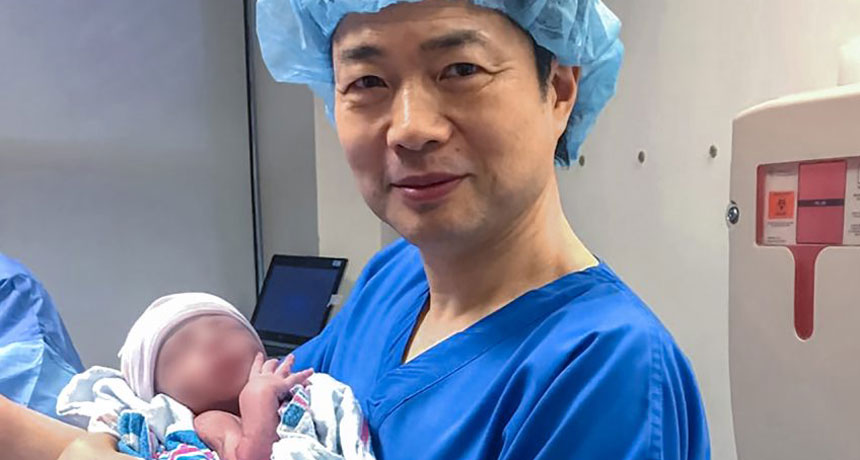
Fertility doctor John Zhang holds a baby boy (whose face has been blurred for privacy). The boy is the world’s first child created by spindle transfer — a technique to replace faulty mitochondria. Such children have been dubbed “three-parent” babies.
New Hope Fertility Center
A baby born in April 2016 may have opened the door to a new world of reproductive medicine. This boy became one of the first intentional “three-parent” babies. The vast majority of this boy’s DNA came from his mother and his father. A small bit of extra DNA came from an unrelated woman. This child got some of his genetic inheritance from each of these adults.
Because of that bonus DNA from the unrelated woman, some people say babies like this boy have three parents.
Scientists didn’t go to all of the effort to mix the DNA from these three people as an experiment. In fact, they did it to overcome a problem in the boy’s mother. That woman had a problem with her mitochondria (MY-toh-KON-dree-uh). These are important little structures — or organelles — present in her cells.
Many cells, including those that make up humans, contain special components that function like little organs. That gives rise to their name, organelles, which actually means little organs. Organelles perform special tasks for their parent cells. And one of the more notable of these organelles is the mitochondrion. Its main job is to help power its cell. To do this, the mitochondria harvest energy contained in the bonds linking atoms in the cell’s fuel (such as glucose). Mitochondria then use that energy to create another molecule, known as ATP (for adenosine triphosphate). That ATP actually serves as the energy source for cells.
Story continues below image
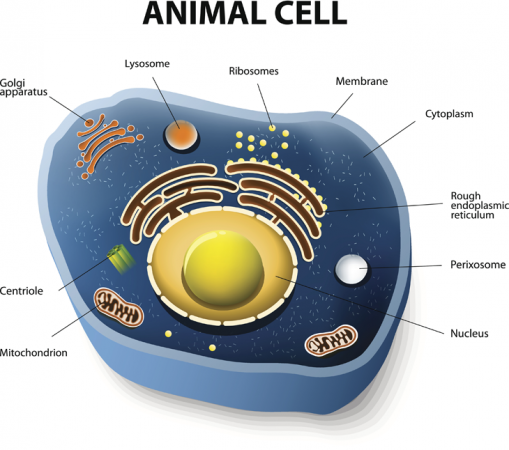
But some of the mitochondria in the boy’s mother have a mutation. That genetic alteration causes Leigh syndrome, a fatal disorder. Most of her mitochondria work properly. That’s why the mom does not have the killer disease. But she can pass on DNA from the faulty mitochondria to her children. And this can put them at risk of Leigh syndrome. Two of her children had already died from the disease. She also had suffered four miscarriages.
It was in hopes of giving this couple a healthy baby that doctors worked to find healthy mitochondria to substitute for her unhealthy ones. Normally, a woman passes on her mitochondria to her offspring through her egg (dad’s sperm don’t contribute any). These organelles also contain a small amount of DNA — just 37 genes. (Most of the roughly 20,000 protein-producing genes needed to make a human are stored in a compartment called the nucleus.) Mutations in some mitochondrial genes most often pose a risk to organs that need lots of energy, such as the brain and muscles. There is no cure or effective treatment for many of these mitochondrial diseases.
The technique used to create the baby boy is new and controversial. His birth, though, caps nearly three decades of work to to produce healthy human eggs by manipulating the organelle. The new baby appears to have been saved from a deadly genetic disease. Still, there are ethical and safety concerns about his three-parent heritage.
And a three-parent baby girl born in January raises even more concerns — in part, just because she is a girl.
Producing healthy babies
Researchers first began swapping mitochondria between egg cells to treat infertility problems almost 20 years ago. Jacques Cohen was one of those researchers.
He’s a scientist who studies human embryos. In the late 1990s, he and colleagues at Saint Barnabas Medical Center in Livingston, N.J., were looking for a way to help women who were unable to have children by in vitro fertilization. Also known as IVF, this process involves taking egg cells from a woman and sperm cells from a man, then incubating them in a dish. Some of those eggs and sperm will combine to form embryos — the first stages of creating a new individual.
Doctors then transfer some of those embryos into the woman’s womb. With luck, one or more will develop into a baby. But some couples’ embryos never developed normally. No one knows why. Cohen’s group thought a dose of cytoplasm — the jellylike “guts” of a cell — from a donor egg might give the implanted embryos a better shot at success.
“Cytoplasm is the most complicated fluid in the universe,” says Cohen. It contains mitochondria, other organelles, proteins and other molecules that do the work of the cell. The mother’s egg normally supplies all the goodies an embryo needs to live for the first few steps of development. But Cohen thought that some of his patient’s eggs might need extra help.
So he extracted 10 to 15 percent of the cytoplasm from an egg donated by another woman. He injected this along with a single sperm cell into a recipient egg. From 1996 to 2001, he performed the procedure 37 times. And this technique proved quite successful. It produced 17 babies for 13 couples!
Cohen later tested eight of the children born this way. Two carried some mitochondria that had come from the donor. That was in addition to some that came from the child’s actual mother. Some of the other six children may have had donor mitochondria at levels too low for his tests to see back then, Cohen now says. But the finding made him curious.
So Cohen and his colleagues tracked down 13 of the 17 children. All were now teenagers. In surveys, their parents said that the kids seemed basically healthy. Cohen doesn’t know whether mitochondria or other parts of the cytoplasm played a role in producing the children. His group stopped performing the technique in 2001 (because of regulatory issues).
Fixing mitochondria
Other scientists have also tried to replace faulty mitochondria more intentionally. The first such attempt in 1983. And it involved mice.
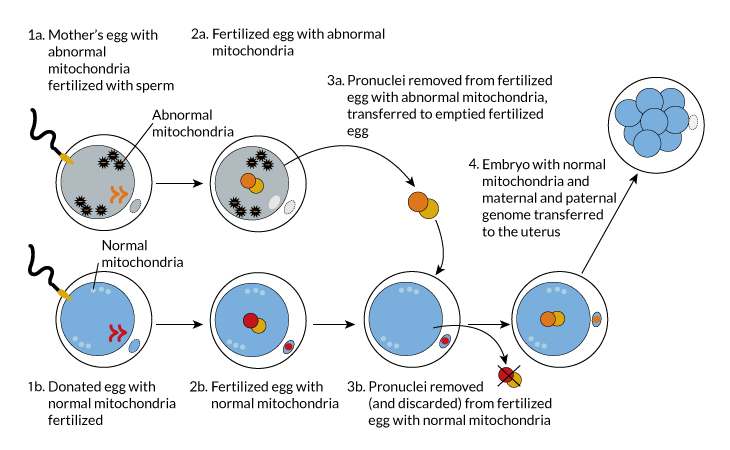
Pronuclei are the central, DNA-containing parts of fertilized eggs. One comes from the egg and another comes from dad’s sperm. At this early stage in development, the two have not yet fused into a single nucleus. (Nuclei is the plural form of nucleus.)
In a technique known as pronuclear transfer, researchers fertilized the mother mouse’s egg and a donor egg at the same time. The pronuclei were removed from the donor’s fertilized egg and discarded. Those from the mother’s fertilized egg were sucked out and then injected into the empty donor egg.
Story continues below image
Pronuclear transfer
Talk of applying this technique in humans promptly raised a few concerns.
Some people claimed that it is not ethical. They argued that it manipulates — maybe even destroys — two embryos.
That’s one issue. Scientists have a more technical one. They note that mitochondria tend to glom onto the nuclei. So unacceptably high numbers of mitochondria from the mother’s egg — including disease-carrying ones — may still find their way into the donor egg, notes Shoukhrat Mitalipov. He is a mitochondrial biologist at Oregon Health & Science University in Portland.
Last June, scientists reported they had refined pronuclear transfer to reduce the number of disease-carrying mitochondria that could enter embryos. Fewer than 2 percent of the mitochondria from the mother’s egg made it into the donor’s egg. But an earlier study suggested that even a half that amount might be dangerous. That’s because mutant mitochondria may copy themselves. Eventually, they might take over the cell and cripple its energy production.
Fertility clinics in the United Kingdom are allowed to use pronuclear transfer to make human babies where there was a high risk of mitochondrial diseases. In fact, none has done so. yet New York fertility doctor John Zhang is involved in the new baby boy’s case. He tried the pronuclear-transfer technique with colleagues at Sun-Yat Sen University of Medical Science in Guangzhou, China. That was more than 10 years ago. Five embryos that were made this way were implanted into a 30-year-old woman. Three grew into fetuses. None, however, survived to birth. Zhang published these results last year in Reproductive Biomedicine Online.
In January 2017, doctors in Ukraine announced that a baby girl was born from this method. Her parents had tried IVF. But, like Cohen’s patients, the couple’s fertilized eggs never grew into an embryo that could be implanted. Instead of adding cytoplasm from a donor egg as Cohen had, fertility doctor Valery Zukin at the Nadiya Clinic in Kiev instead used pronuclear transfer. And they report success — a baby girl.
Labs in Ukraine and Germany confirmed that most of the baby’s DNA is from her mother and father. Only her mitochondrial DNA comes from an egg donor. Zukin used the same technique again. Another couple is now expecting a baby boy next month.
Some people are concerned that these babies might have health problems later. Some people also may see this as an ethical problem. Why? The technique was not used to prevent mitochondrial diseases, but instead as a type of fertility treatment.
Marcy Darnovsky is one of the critics. She is executive director of the Center for Genetics and Society in Berkeley, Calif. Doctors such as Zukin are selling unproven and possibly dangerous services to customers, she charges. “This is the ugly face of commercial and status incentives driving unscientific human experimentation,” she said in statement about the baby girl’s birth.
Dividing cells
Doctors used a different technique — spindle transfer — to produce the baby boy born last April. The body’s genes reside in the DNA found in the body’s 46 different chromosomes. When a cell divides to create egg or sperm cells, it splits those 46 chromosomes into two equal sets of 23. To get portioned out properly, those chromosomes attach themselves to protein fibers. Those fibers are known as spindles. The new transplant technique gets its name from those fibers.
The technique starts with two unfertilized egg cells. One comes from the mother and the other from a donor. In both cells, a membrane surrounding the nucleus has broken down. The spindle in each has not, however, completed a separation of the chromosomes.
Researchers remove the spindle and its attached chromosomes from the donor egg and discard them. Then they do the same to the mother’s egg — except that they keep her spindle and chromosomes. These they inject into the donor’s nearly empty egg. Then the researchers add the dad’s sperm cell into this egg to fertilize it.
Story continues below image
Spindle transfer
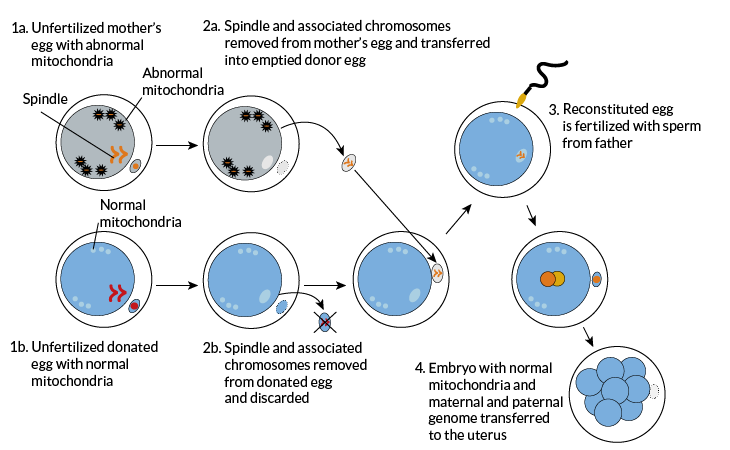
Mitalipov in Portland pioneered spindle transfer. In 2009 he showed that he could produce healthy baby monkeys with it. Those experiments showed that fewer of the mom’s mitochondria made it into the donor egg than with pronuclear transfer. Typically, the carryover amounted to 1 percent or less.
But Mitalipov would like to do even better. “This 1 percent is haunting us,” he says.
Spindle transfer has another possible downside: Chromosomes may fall off the spindle. That could result in an embryo with too few chromosomes — or too many if some are left in the egg from the donor. Both cases usually result in abnormal development. Of the five embryos on which Zhang performed spindle transfer, only one developed normally. That was the baby boy born last April.
Tests reportedly found that he has 1 percent of his mom’s mitochondrial DNA. At 3 months old, he appeared healthy. What his health will look like, long-term, however, is unknown. Besides the risk of even trace levels of mitochondria ballooning, another study suggests that the child’s health, over time, might be affected by mismatches between the parents’ nuclear DNA (which is not from mitochondria) and the donor’s mitochondrial DNA.
A controversial birth
Some researchers take issue with the moniker “three-parent” baby. Cohen, for one, says the term is wrong. Mitochondrial DNA does not contribute to a person’s traits. So, he argues, the person who donates mitochondrial DNA is hardly a “parent.”
Andrew R. La Barbera agrees. He is chief scientific officer of the American Society for Reproductive Medicine. “A person’s essence as a human being comes from their nuclear genetic material,” he says, “not their mitochondrial genetic material.” So children conceived using mitochondrial transfer have just two parents, he maintains.
But there are bigger controversies here than what makes a parent. Opponents of these techniques worry that none has been fully tested.
Darnovsky says, “We wish the baby and family well, and hope the baby stays healthy.” But until these techniques are shown to be safe, she says, “I have a lot of concerns about this child and about future efforts to use these techniques.”
Zhang also drew fire for going to Mexico to perform the procedure. In America, researchers are banned from doing things that could alter human DNA in a way that can be passed from generation to generation. Spindle and pronuclear transfer both do this. The worry is that genetic changes of future generations won’t stop with preventing diseases. Policy makers wanted to outlaw efforts to make genetically enhanced “designer babies.”
However, a panel of experts said in February 2016 that it is ethical to make three-parent baby boys. But not girls. Why? Fathers almost never pass mitochondria on to their babies. So baby boys born through such techniques should never pass along the donor’s mitochondria.
A baby girl, though? That would be a very different story.







