Explainer: The making of a snowflake
Here’s the science behind a snowflake’s striking shape

Snowflakes take a long journey down from the clouds in which they form. But it takes more than cold air and moisture to create them.
SbytovaMN/iStockphoto, adapted by L. Steenblik Hwang
Snowflakes come in an infinite range of shapes and sizes. Many appear to be two-dimensional works of art. Others look like a matted cluster of fraying ice strands. Most come as individuals, although some can fall as multi-flake clumps. What all have in common is their source: clouds that usually hover at least a kilometer (0.6 mile) above the ground.
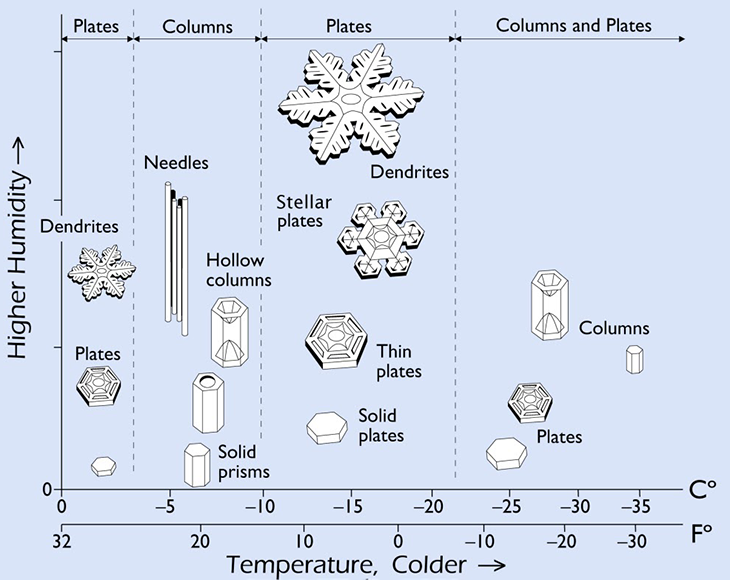
In winter, the air up there can be very cold — and will get chillier the higher you go. To form snowflakes, those clouds need to be below freezing. But not too cold. Snowflakes form from the moisture in a cloud. If the air gets too cold, a cloud won’t hold enough water for anything to precipitate out. So there has to be a balance. That’s why most flakes develop at or just below freezing — 0º Celsius (32º Fahrenheit). Snow can form in cooler environments, but the colder it gets, the less moisture will be available to make a snowflake.
In fact, a cloud’s air has to be supersaturated with moisture for a flake to form. That means there is more water in the air than would normally be possible. (The relative humidity can reach 101 percent during supersaturation. That means there is 1 percent more water in the air than it should be able to hold.)
When there is too much liquid water in the air, a cloud will try to rid itself of the excess. Some of that excess can flash freeze into crystals, which then lazily meander to the ground.
Or that’s the simple answer. The details aren’t quite that straightforward.
Cold water alone won’t a snowflake make
One more thing is needed to turn cloud moisture into a flake. Scientists call it a nucleus (NOO-klee-uhs). Without something to glom onto, water droplets can’t freeze. Even when the air temperature is well below freezing, water droplets will remain liquid — at least until they have a solid object onto which they can attach.
Usually, that will be something like a pollen grain, dust particle or some other airborne bit. It could be smoglike aerosols or the volatile organic compounds released by plants. Even tiny soot particles or microscopic metal bits spewed in a car’s exhaust could become the nuclei around which snowflakes crystalize.
Indeed, when the air is very clean, it can be very difficult for a cloud’s moisture to find a nucleus.
Near the ground, any object can prove a suitable freeze-onto zone. That’s how we get rime ice to form on the branches of trees, light poles or vehicles. Different from frost, rime ice develops when supercooled water droplets freeze onto subfreezing surfaces. (In contrast, frost forms when moisture collects on surfaces in liquid form, and then freezes.)
High in a cloud, there have to be some tiny floating particles for snow crystals to develop. When the right conditions do emerge, supercooled drops of water will latch onto these nuclei (NOO-klee-eye). They do it one by one, building an ice crystal.
How the flakes shape up

To understand what’s behind a snowflake’s intricate and complex shape, scientists turn to chemistry — the action of atoms.
A molecule of water, or H2O, is made of two hydrogen atoms bound to an oxygen atom. This trio combines into a “Mickey Mouse” pattern. That’s due to polar covalent (Koh-VAY-lent) bonds. The term refers to three atoms that each share electrons with one another, but unevenly.
The oxygen’s nucleus is larger, so it has more pull. It yanks more strongly at the negatively charged electrons that they share. This brings those electrons a little closer. It also gives the oxygen a relative negative electric charge. The two hydrogen atoms end up a tad positive, in terms of charge.
Alone, the structure of a water molecule resembles a wide V. But when multiple H2O molecules find themselves close to one another, they begin to pivot so that their electrical charges pair up. Opposite charges attract. So a negative hydrogen aims itself towards a positive oxygen. The shape that tends to result: a hexagon.
That’s why snowflakes have six sides. It stems from the hexagonal — six-sided — structure of most ice crystals. And hexagons team up. They link with other hexagons, growing outward.
That’s how a snowflake is born.
Each hexagon contains a lot of empty space. This explains why ice floats on water; it’s less dense. Warmer H2O molecules in the liquid phase are too energetic to settle into a rigid hexagon. As a result, the same number of H2O molecules occupy 9 percent more space as solid ice than they do as liquid water.
Depending on the temperature, these hexagons join with each other and grow in different ways. Sometimes, they make needles. Others may form branch-like dendrites. All are beautiful. And all have their own unique story of crystal growth.
Snowflake structure has been a scientific curiosity since Wilson Alwyn “Snowflake” Bentley attached a microscope to his camera in 1885 and became the first person to photograph them.
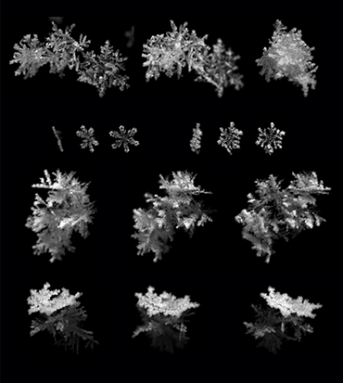
These short-lived crystals still enthrall scientists. To better capture their shape and movement, Tim Garrett at the University of Utah in Salt Lake City recently built a better snowflake camera. He’s been using it to get an inside view of the variety of flakes that fall.
Snowflakes by the numbers
1. A typical snowflake may contain 1,000,000,000,000,000,000, or one quintillion water molecules. That’s a million times a million times a million! Those building blocks can configure themselves in a virtually infinite array of patterns. So it stands to reason that no two snowflakes that you encounter will ever be exactly the same.
2. Snowflakes tend to be less than a coin’s width in diameter. But once in a while, true whoppers form. In January 1887, a Montana rancher reported snowflakes “larger than milkpans.” That would make them some 38 centimeters (15 inches) across. As that was back before portable home cameras, this number can be challenged. But snowflakes larger than 15.2 centimeters (6 inches) do sometimes develop. Biggies tend to form when temps are near freezing and the air humid. A snowflake’s size also reflects other factors. These include wind speed and direction, dew point — even how electrified different layers of the atmosphere are. But nobody has ever really conducted measurements when gigantic flakes were flying.
3. Most snowflakes fall at roughly a walking pace — between 1.6 and 6.4 kilometers (1 and 4 miles) per hour.
4. With the cloud in which flakes form usually one to two kilometers (0.6 to 1.2 miles) up, each crystalline wonder may drift anywhere from 10 minutes to more than an hour before reaching the ground. Sometimes, they get carried back up, and it takes several tries for them to reach the ground.







