New glue offers to turn any small walking robot into Spider-Man
The glue’s superpower: electric fields that turn its stickiness on or off
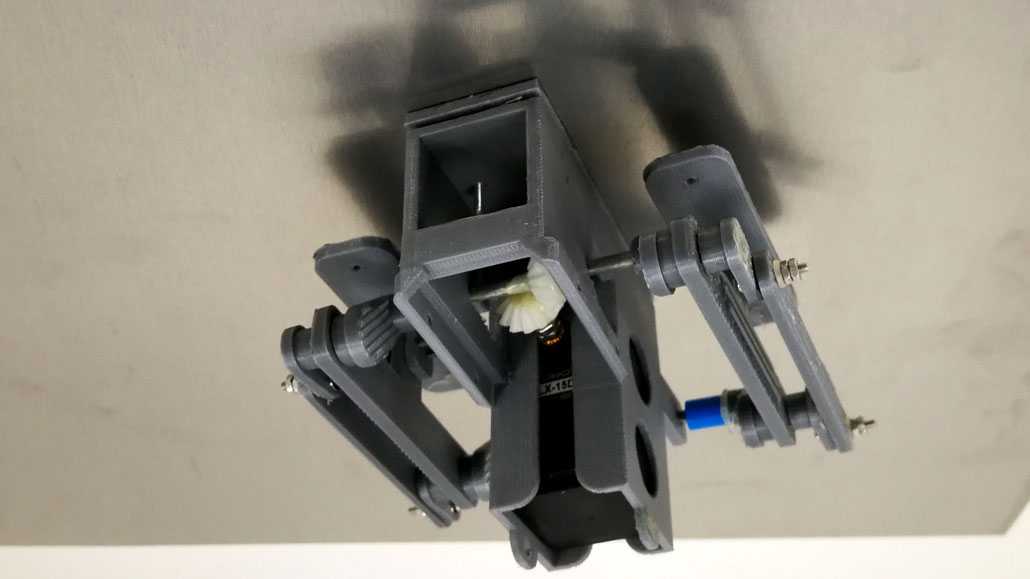
Is this a lizard on the ceiling? An insect? Spider-Man? No, it’s a climbing robot!
Lizong Dai/Xiamen Univ.
By Shi En Kim
For a robot to climb a wall, it must latch onto it and controllably stick. That’s not hard. Many glues can hold an object to some surface. What those glues typically can’t do, however, is later unstick and restick on command. And do it over and over with each climbing step. Researchers in China have now conquered that problem. Like Spider-Man, nearly any small, walking robot might be able to defy gravity through use of this glue.
Fancy chemistry and a periodic electric jolt control the robot’s stickiness. The researchers shared details of how all this works April 28 in Science Robotics.
Conghui Yuan and Lizong Dai are polymer scientists. They developed their new climbing robot at Xiamen University. It’s on China’s southeast coast. Polymers are long chain-like molecules that are made up of smaller repeating links. Yuan and Dai padded the soles of their robots’ feet with a water-loving hydrogel. This polymer gave their robots climbing superpowers.
That hydrogel had been inspired by clingy mussels.
Adult mussels stay rooted to one spot their whole lives. While battered by waves or strong currents, the shellfish firmly hold onto rocks in rivers and oceans. To do this, mussels secrete a gluey protein. It contains a natural polymer that includes catechol (KAT-eh-koal). This polymer can bind strongly to all types of surfaces. The researchers’ added a catechol-based substance to their hydrogel so that it can glom onto surfaces as a mussel does.
To turn off the catechol’s stickiness, the researchers shielded the chemical with a non-sticky molecule. Under acidic conditions, that protective cap falls off to expose the sticky, catechol-based substance. The protective cap pops back on in a basic (or alkaline) environment. So the hydrogel gets sticky in acidic conditions and loses its stick in basic ones.
Electric zaps control the stickiness
Altering the glue’s pH turns its stickiness on and off. And here’s where electricity comes in. Giving a little jolt breaks the hydrogel’s water down into hydrogen and oxygen. This process is known as electrolysis. The researchers turn this on using a battery.
Different chemical reactions occur at the battery’s positive and negative electrodes. Here they are metal connectors that deliver electricity to the hydrogel. Reactions at the negative electrode release hydroxide ions (OH–). Their presence turns the water basic. At the positive electrode, the hydroxide ions get used up. This makes the water here acidic, upping the hydrogel’s stickiness.
To make the glue unsticky, the researchers switch the direction of their system’s electric field. This reverses the polarity of the electrodes. The positive one now becomes negative and vice versa.
One can think of the hydrogel pads on the robot’s feet as shoes. Controlling the electric field on the sole of each shoe determines whether a climbing foot will stick — or let go.
There’s a big advantage to using electricity as the way to alter the hydrogel’s pH, says Dai. Normally, he says, “it’s very hard to change the pH of the environment.” For each step a robot took, a chemical system would need to keep pumping acidic and alkaline compounds into the hydrogel shoes.
Clearly, argues Yuan, using electricity is much simpler.
As the researchers cycle through the application of positive and negative electric fields, the feet alternately adhere and come free. This allows the team’s new palm-sized robots to climb. They can roll or clomp upward — or even upside down. But they’re not speedy. They advance at a caterpillar’s pace.
Credit: Lizong Dai, Xiamen University
New glue has its limits
While the reversible hydrogel is a true glue, it’s less sticky than cellophane tape. Right now, robots shoed with it can only climb metal or some other electrically conducting surface (because such surfaces need to act as electrodes). But the robots’ ultimate weakness is that its feet need to stay very moist. That moisture is what feeds the hydrogel’s electrochemical reactions.
Engineering, however, can overcome those roadblocks, the researchers say. Embedding metallic wires in the hydrogel might replace conducting surfaces as the electrode, says Yuan. This would allow the robots to climb non-conducting surfaces. To get around drying out, his team is mixing carbon-based solvents into the hydrogel’s water. Such organic solvents slow the speed at which the water dries.
“There’s room for improvement,” agrees Amy Kyungwon Han. She’s a mechanical engineer at Stanford University in California. Still, she says that the new hydrogel’s existing traits are impressive.
“You can control the adhesion,” notes Han, who wasn’t involved in the study. And the hydrogel is “self-healing. It can also adhere to wet surfaces,” she adds. “All these are great, because each point is very difficult [to achieve].”
Perhaps Dai and Yuan’s robots are best suited for spots similar to the homes of the mussels that inspired them: underwater. Few polymers stay sticky when wet. But Dai and Yuan’s glue needs water. The researchers hope to succeed in one day making climbing underwater robots. These could be useful for moving through submerged caves or around undersea pipe networks.
The researchers also are open to designing a suit made from the hydrogel. Why? “We have a dream,” they say, “to make people climb like Spider-Man.”
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.







