New recycling technologies could keep more plastic out of landfills
A lot of plastic is impossible to recycle. Chemists are trying to change that

Much of the plastic we use goes into landfills because the material is too difficult to recycle into useful new products.
Abdul Raheem Mohamed/EyeEm/Getty Images
It feels good to recycle. When you sort soda bottles and plastic bags from the rest of your garbage, it seems like you’re helping the planet. The more plastic you put in the blue bin, the more you’re keeping out of landfills, right?
Wrong. No matter how much plastic you try to recycle, most ends up in the trash heap.
Take flexible food packages. Those films contain several layers, each made of a different type of plastic. Because each type must be recycled separately, those films are not recyclable. Even some items made from only one kind of plastic are not recyclable. Yogurt cups, for instance, contain a plastic called polypropylene (Pah-lee-PROH-puh-leen). When this gets recycled, it turns into a gross, dark, smelly material. So most recycling plants don’t bother with it.
Only two kinds of plastic are commonly recycled in the United States. One is the type used in soda bottles. That’s called PET, short for polyethylene terephthalate (Pah-lee-ETH-uh-leen TAIR-eh-THAAL-ayt). The other is the plastic in milk jugs and detergent containers. That’s high-density polyethylene, or HDPE. Together, those plastics make up only a small fraction of plastic trash. In 2018 alone, the United States landfilled 27 million tons of plastic, according to the U.S. Environmental Protection Agency. A mere 3 million tons was recycled.
Low recycling rates aren’t just a problem in the United States. Only 9 percent of all the world’s plastic trash has ever been recycled. Twelve percent was burned. Seventy-nine percent has piled up on land or in waterways. Researchers reported those estimates in 2017 in Science Advances.
Good news/bad news
The amount of plastic recycled in the United States has increased over the last few decades. However, those levels still pale in comparison with the amount of plastic that goes into landfills.
Plastic waste management, 1960–2018
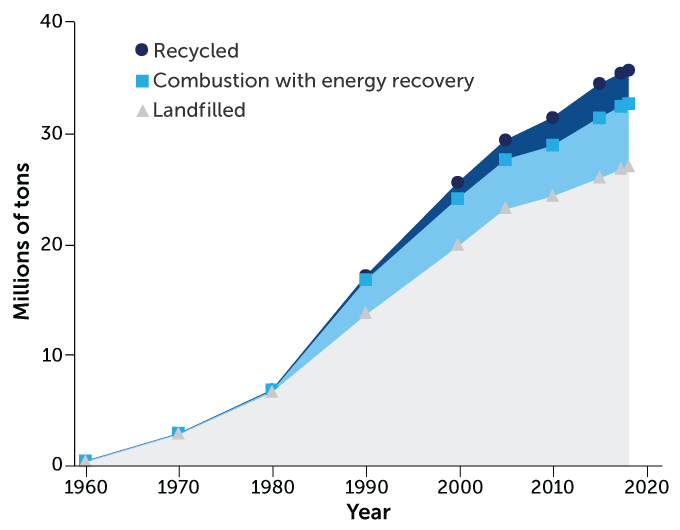
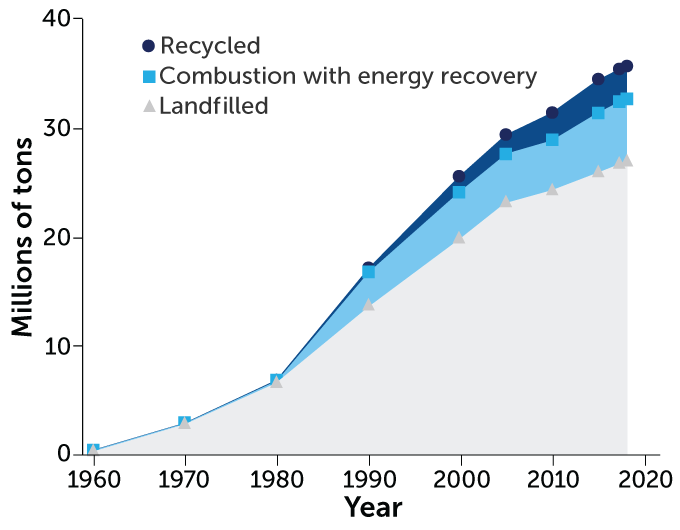
Even when plastic does get recycled, it isn’t good for much. Recycling changes the consistency of a plastic. So recycled plastics have to be mixed with brand-new material to make sturdy products. What’s more, recycling a bunch of different colored plastic together creates a dark mixture. That means a lot of recycled plastic can only be used to make items whose color doesn’t matter, such as benches and dumpsters.
Plastic recycling clearly has a lot of room for improvement. And with plastic piling up everywhere from mountaintops to the seafloor, there is an urgent need for better recycling. Luckily, chemists around the world are on the case. Some are trying to make it easier to recycle more types of plastic. Others are trying to turn recycled plastic into more useful products. Both strategies could cut how much plastic winds up in landfills or oceans.
Picking plastics apart
One big challenge to recycling is that every type of plastic must get processed separately. “Most plastics are like oil and water,” says Geoffrey Coates. He’s a chemist at Cornell University in Ithaca, N.Y. Plastics just don’t mix, he says. Take, for example, a detergent container. The jug may be HDPE plastic, but its cap is polypropylene. What would happen if a recycling plant melted those two plastics together and tried to make a new jug from the blend? “It would basically crack down the side,” Coates says. “It’s crazy brittle. Totally worthless.”
That’s why all the plastic in the recycling bin first goes to a material recovery facility. There, people and machines sort trash. Sorted plastics are then washed, shredded, melted and remolded. The system works well for simple items like soda bottles and milk jugs. But it doesn’t work for items like deodorant containers. A deodorant bottle, cap and crank could all be different plastics.
Food packaging films made of different plastic layers are even harder to take apart. Every year, 100 million tons of these films are made worldwide. Those films all go to landfills, says George Huber. He’s a chemical engineer at the University of Wisconsin–Madison.
Huber and his colleagues came up with a way to sort these pesky mixes of plastics. The researchers use different liquids to dissolve different plastic parts off an item. The trick is choosing the right liquids to dissolve only one plastic at a time, Huber says. This strategy was described last November 18 in Science Advances.
False advertising
Many plastic products are labeled with a number inside a triangle that symbolizes recycling. Yet, only plastics with 1 (polyethylene terephthalate) or 2 (high-density polyethylene) are widely recycled in the United States. The rest typically get buried in landfills.
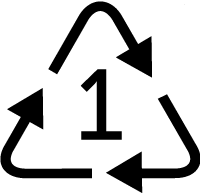
PET
Water and soft drink bottles, salad domes, cookie trays, salad dressing and peanut butter containers

HDPE
Milk and juice bottles, freezer bags, shampoo and detergent bottles

PVC
Cosmetic containers, commercial cling wrap

LDPE
Squeeze bottles, cling wrap, trash bags

PP
Microwave dishes, ice cream tubs, yogurt containers, detergent bottle caps

PS
CD cases, plastic disposable cups, plastic cutlery, video cases

EPS
Foam polystyrene hot drink cups, food takeaway trays, protective packaging for fragile items
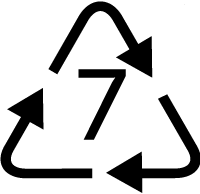
Other
Water cooler bottles, flexible films, multimaterial packaging
Huber’s team tested the technique on a food-packaging film. The film contained three plastics: polyethylene, PET and ethylene vinyl alcohol, or EVOH. The researchers first stirred the film into a liquid called toluene (TAHL-you-een). That dissolved the polyethylene layer. Then, Huber’s team dunked the film in another chemical to strip off the EVOH. The researchers plucked out the remaining PET film and set it aside. To recover the other two plastics from the liquids, the researchers mixed in “antisolvent” chemicals. These chemicals caused the plastic molecules drifting in the liquids to clump together so that they could later be fished out.
Making plastics mix
There may be shortcuts to recycling unsorted mixes of plastics. Chemicals called “compatibilizers” help different plastics blend. There is no chemical that allows every type of plastic to mix. But Coates’ team has made one to combine polyethylene and polypropylene. That could make recycling much easier. Those two plastics make up the bulk of the world’s plastic trash.
The new compatibilizer contains specially designed molecules. Each molecule has four pieces. Two pieces of polyethylene alternate with two pieces of polypropylene. Those segments latch on to plastic molecules of the same kind in a mixture. It’s as if polyethylene were made of Legos, and polypropylene were made of Duplos. The compatibilizer molecule is like a connector that fits both types of blocks. That helps polyethylene and polypropylene molecules link up. The researchers reported this work in 2017 in Science.
The first test of this compatibilizer involved using it as a glue. Coates’ team spread a layer of the chemical between a strip of polyethylene and a strip of polypropylene. Then, the researchers tried to peel the plastics apart. The two plastics would normally separate easily. But with the glue between them, the plastic strips broke before the seal.
The researchers also mixed their compatibilizer into a melted blend of the two plastics. Adding just 1 percent of the new chemical created a tough plastic product.
Good as new
Making it easier to recycle plastic isn’t enough. To reuse the same material over and over, recycled plastic needs to be as good as new. Right now, it’s second-rate.
One problem is all the extra chemicals in plastic trash. Plastic items often contain dyes, flame retardants and other additives. Current recycling cannot get rid of those contaminants. As a result, recycled plastic comes with lots of impurities. Few manufacturers can use plastic with a random mishmash of properties to make something new.
Plus, recycling breaks some of the chemical bonds in plastic molecules. That affects the strength and consistency of the material. Recycling plastic is sort of like reheating pizza in the microwave. You get out pretty much what you put in, just not as good. That limits the number of times plastic can be recycled.
The solution to both problems could be a new type of recycling, called chemical recycling. This process takes plastic apart at the molecular level. And it could make pure new plastic an infinite number of times.
The molecules that make up plastics are called polymers. Those polymers, in turn, consist of smaller building blocks called monomers. Heat and chemicals can break polymers down into monomers. Those monomers can then be separated from dyes and other contaminants. Piecing pure monomers back together creates plastic that’s good as new.
“Chemical recycling has really started to emerge as a force … within the last three or four years,” says Eric Beckman. He’s a chemical engineer at the University of Pittsburgh in Pennsylvania. But most chemical recycling requires a whole lot of energy. “It’s not ready for prime time,” he says.
Some plastics are easier to chemically recycle than others. “The one that’s farthest along is PET,” Beckman says. That’s the plastic in water and soft-drink bottles. “That polymer happens to be easy to take apart.” Several companies are trying to chemically recycle PET. One is a French company called Carbios.
Carbios breaks down PET using a molecule called an enzyme. Microbes in compost naturally produce this enzyme to decompose the waxy coating on leaves. But the enzyme is also good at breaking apart PET. “The enzyme is like a molecular scissor,” says Alain Marty. He is the chief scientific officer at Carbios in Saint-Beauzire, France. The enzyme snips PET polymers into their two monomers. One is ethylene glycol. The other is terephthalic acid.
Because this enzyme evolved to decompose leaves, not plastic, it’s not perfect. But Marty’s team improved it by redesigning its “active site.” That’s where the enzyme latches onto PET. The redesign involved swapping out some amino acids at the active site for others. Marty and his colleagues reported how they did this April 8, 2020 in Nature.
The researchers tested their upgraded enzyme on plastic flakes from colored PET bottles. About 90 percent of the plastic broke down in about 10 hours. The team purified the PET monomers and used them to make new clear plastic bottles. Those bottles were just as strong as the originals. Carbios is now building a plant in France to chemically recycle PET.
Microbial help
In one study, an enzyme naturally produced by microbes broke down about 50 percent of polyethylene terephthalate, or PET (blue line). A tweaked version of the enzyme broke down more than 80 percent of the plastic (black dotted line). Increasing the amount of the enzyme from 1 milligram per gram of PET to 3 milligrams made it even more efficient — breaking down about 90 percent of the plastic.
PET breakdown by an enzyme
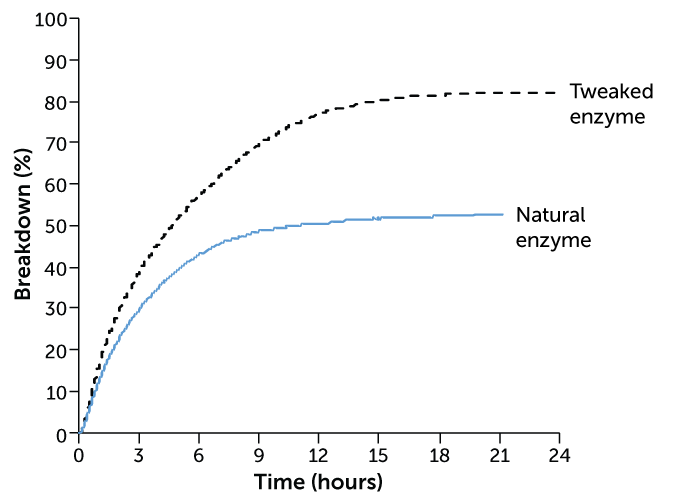
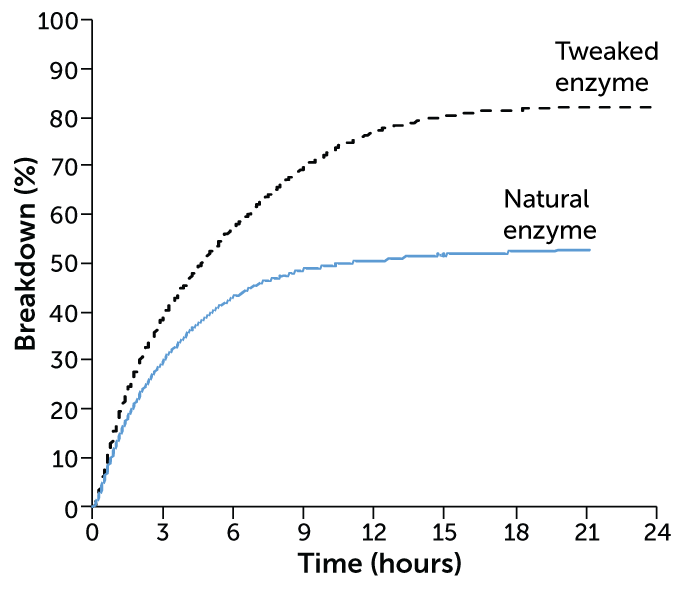
Milder conditions
PET is a special case for chemical recycling. Other plastics are much harder to break down. Taking apart polyethylene, for instance, requires temperatures over 400° Celsius (750° Fahrenheit). At such high heat, the chemistry is chaotic. Plastic molecules break down randomly. The result is jumble of different compounds. Those molecules can be burned as fuel but not used to make new plastic.
Susannah Scott proposes a better way to recycle polyethylene. A chemist, Scott works at the University of California, Santa Barbara. Her idea is to break the plastic down under milder conditions. That more-controlled breakdown could produce ingredients for soaps and other products. These ingredients are called alkylaromatic (AL-kyl-air-oh-MAT-ik) compounds.
To make these, Scott placed polyethylene inside a chemical-reaction chamber. Then she heated it up to 280 °C (540 °F). Her team cooked the plastic with a powder that contained tiny platinum particles for 24 hours. The platinum helps break the carbon-hydrogen bonds in the polyethylene. That process releases hydrogen. The hydrogen then helps break the plastic’s carbon-carbon bonds. This chops the plastic molecules up into smaller pieces. Each piece is about 30 carbon atoms long. Those fragments arrange themselves into rings. Then voilà — alkylaromatic compounds.
Scott and her colleagues tested this technique on a plastic bag and a bottle cap. Both were made of polyethylene. About 69 percent of the plastic bag became liquid containing alkylaromatic compounds. About 55 percent of the bottle cap was converted. Scott’s team reported these results last October 23 in Science. Transforming the polyethylene also produced gases like methane. Those gases could be burned as fuel to heat the reaction chamber.
Built to last
Plastics were never designed to be used more than once. That’s why recycling them is so hard. But some researchers are now asking, “What does the next generation of materials look like? How do you design a material specifically so that it never has to go into a landfill?” Beckman asks. The new goal, he says, is to design a plastic “that falls apart on command.”
A new generation of plastics called PDKs may be infinitely recyclable. PDKs, short for poly(diketoenamine)s, were first described in Nature Chemistry in 2019.
“PDKs have the ability to break their bonds under relatively mild conditions,” says Brett Helms. He’s a chemist at the Lawrence Berkeley National Laboratory in California. Simply dunking a PDK in acid breaks it into its monomers.
The plastic must be dunked in a very acidic liquid to decompose. Say, a pH of 1 or 2. “Materials don’t usually encounter a pH that’s that low,” he notes. “It’s not like if you put PDKs in vinegar, the polymer is going to start breaking down.” But it could make for easy recycling. PDK monomers could then be used to make pristine new plastic, again and again.
The plastics used today are so cheap that it would be hard for any new plastic to compete solely on cost, Beckman says. For now, infinitely recyclable plastic is just a scientific curiosity. But someday, plastics made to be recyclable from the get-go may help solve the world’s plastic waste problem.







