New rules point scientists toward next-gen germ-killers
Chemists uncover the tricks compounds use to move inside many bacteria
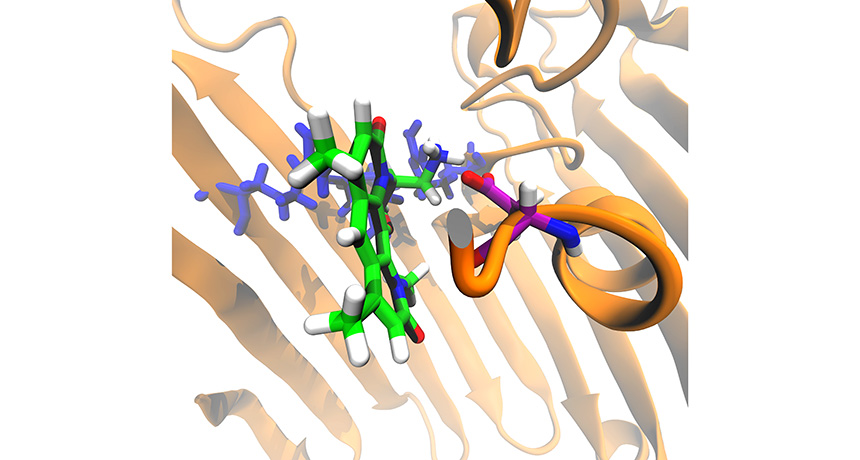
Chemists tweaked the structure of an antibiotic (mostly green and white). The new compound can travel into the cell through a channel (brown) on the cell surface.
M. F. Richter et al./Nature 2017
Like fortress walls, the membranes that encase bacteria are hard to breach. Not many chemical compounds can break through these defenses to do battle with these germs. But some compounds do. By analyzing them, researchers now know what it takes. Drug developers might want to use the newfound rules to develop more and better antibiotics to treat bacterial infections.
Escherichia coli is one bacterium that belongs to a type known as gram negative. (They got their name because they don’t turn purple when exposed to a violet dye used for what is known as “gram staining.”) These bacteria have two membranes. These barriers control which chemicals enter and exit the germs.
Not all germs are dangerous. But doctors often prescribe antibiotic medicines for pathogens, which are germs that cause disease. Alas, most antibiotics can’t get inside of the outer of a gram negative’s two membranes, notes Paul Hergenrother. He’s a chemical biologist at the University of Illinois at Urbana-Champaign. And many drugs that have killed gram-negative bacteria in the past no longer can be counted on to do so. The reason: These germs have evolved traits that allow them to resist — ignore — the drug’s ability to poison them.
The World Health Organization is part of the United Nations. It has been encouraging scientists to develop alternative drugs. In February it released a list of pathogens that not only are resistant to multiple drugs but also that threaten human health. Many bacteria on that list are gram negatives.
To develop new drugs against these germs, researchers would like to understand exactly how successful drugs cross into these one-celled microbes. In the past, researchers have studied how a bacterium’s outer barrier — cell membrane — works, explains Kim Lewis. He’s a microbiologist at Northeastern University in Boston and wasn’t involved in the new study.
With the growing problem of drug-resistant bacteria, Hergenrother and his team decided to go about developing germ killers differently. Explains Lewis: They decided to attack the problem by asking what allows those chemicals to cross through the barrier. That approach pointed them to little channels, or pores, that cut through the cell walls. The term for these little pores: Porins
The Illinois scientists shared what they learned online May 10 in Nature.
Developing some ‘rules’
The outer membrane of gram-negative cells is dotted with proteins. These are those channel-like porins. They allow nutrients to pass through and feed the bacteria. However, Hergenrother’s team found, those porins also can allow drugs to enter the pathogens.
The Illinois researchers investigated what allows chemicals to enter cells through those porins. To test this, they built 100 different compounds. Each mimicked some natural chemical that is able to kill bacteria. Then the researchers mixed each compound in a test tube for 10 minutes with a lot of E. coli. Afterward, they measured how much of each compound had snuck into the cells.
A dozen of the novel chemicals succeeded and began accumulating in the germs. What feature did they have in common? All contained an amine (AA-meen) group, a collection of atoms that contains the element nitrogen.
Next, the team collected a larger set of compounds. All contained amine groups. Each was tested in a similar setup to what had been used with the novel compounds. Afterward, the scientists measured whether these new chemicals penetrated the E. coli and collected inside them. Such a buildup is a sign the compound might also work as a drug.
With these data, the researchers turned to a computer model to learn more. They asked it to probe other features of a chemical’s structure or recipe that appear to have helped it get into cells. This analysis showed that stiff, flat molecules seemed to work better than flexible, round ones. That makes sense, notes Hergenrother. It’s harder to put a basketball through a narrow opening than to slide a ruler through it.
From this, the researchers developed a set of rules on how compounds penetrate gram-negative bacteria. To then test those rules, the researchers turned to a natural antibiotic. It has a tongue-twister name: deoxynybomycin (De-OX-ee-ny-boh-MY-sin). This drug works on only the gram-positive bacteria.
Unlike the gram negatives, these germs have just one cellular membrane. Deoxynybomycin is flat and rigid. So it already had the right shape to slip through a porin, notes Hergenrother. By adding an amine group to the drug, the team thought it could make it kill gram-negative bacteria, too.
And it worked.
The researchers tested this new compound against gram-negative pathogens that are resistant to many germ-killing drugs. The altered drug successfully killed all but one of them.
These new findings could help researchers convert other antibiotics that work against gram-positive drugs into ones that also can quash gram-negative germs, Hergenrother predicts.







