New ways to clean up polluted sources of drinking water
Some succeed with hard-to-remove pollutants, others would lower the cost of treating water

Researchers are testing new ways to bring affordable water treatment to smaller towns and to people who use well water.
pinkomelet/iStockphoto
David Reckhow is an engineer at the University of Massachusetts Amherst. A large shed on the outskirts of town has become his world-class laboratory. That’s why many experts want to use this lab — more than the building has space for. These people want to test their new technologies to clean up drinking water.
To tackle the lab’s popularity, he’ll roll out a new annex next year — one on wheels. This Mobile Water Innovation Laboratory will take promising new and affordable technologies to local communities for testing.

U.S. drinking water is heavily regulated. Overall, it also tends to be pretty clean. Still, several recent water-poisoning cases have grabbed national headlines. Probably the most well-known was the 2014 lead crisis in Flint, Mich. Lead is a toxic, heavy metal that has been used in many water pipes around the nation. Studies have shown it can sicken people and permanently lower a developing child’s IQ. A change in how Flint’s drinking water was treated exposed an estimated 99,000 city residents — many of them children — to elevated levels of lead.
Such events pointed to ongoing weaknesses in how some water is treated. It also shook up the trust of many people in their tap water.
Flint was not an isolated water crisis. In just the 2013 to 2014 period, 42 outbreaks of drinking water poisonings were recorded by the U.S. Centers for Disease Control and Prevention, or CDC. These outbreaks led to more than 1,000 people getting sick. Thirteen died. The top culprits were Legionella bacteria and some form of chemical, toxin or parasite. CDC reported these data in the November 10, 2017 Morbidity & Mortality Weekly Report.
But such numbers tell only part of the story. Many pollutants that the U.S. Environmental Protection Agency regulates cause problems only when people are exposed for months to years. For instance, the effects of lead don’t tend to show up right away. One study last February surveyed records from 1982 to 2015 of drinking water that didn’t meet EPA’s standards. (Those were the most recent years for which data were available.) It found that 21 million people were served by drinking water systems that failed U.S. standards. Researchers reported their findings in the Proceedings of the National Academy of Sciences.
All of this helps explain why there is so much interest in ways to better disinfect drinking water, filter out poisons and detect when water-treatment slipups took place.
Current technology can remove most pollutants, says David Sedlak. He’s an environmental engineer who works at the University of California, Berkeley. Those pollutants include microbes, arsenic, nitrates and lead. He points to others, however, “that are very difficult to degrade or transform.” These include industrial chemicals, such as the ones used to make water- and stain-repellent treatments for fabrics and more.
Smaller communities, especially, can’t always afford top-of-the-line equipment to pull out challenging pollutants. Many also can’t afford to replace leaking or lead-based pipes. So Reckhow’s facility is testing new, more affordable approaches to help such communities.
Some researchers are adding technologies to deal with new and potentially harmful pollutants. Others are designing approaches that work with existing water systems. Still others aim to clean up pollutants at their source.

New tech solutions
Reckhow’s team at UMass Amherst is testing ferrate as a replacement for several water treatment steps. As an electrically charged form of iron, ferrate is an ion. This material kills bacteria in the water. But it also had an added benefit. It breaks down carbon-based pollutants into less harmful chemicals.
Finally, ferrate makes ions of the metal manganese less soluble in water. That will make them easier to filter out, say Reckhow and his colleagues. They described the treatment in a 2016 paper in Journal–American Water Association.
With its many benefits, ferrate might help streamline the drinking-water treatment, says Joseph Goodwill. He’s an environmental engineer who works at the University of Rhode Island in Kingston. Ferrate might also cut the need for disinfectants. Some of these, such as chlorine, can yield dangerous by-products, he notes.
Some water-treatment plants use ozone gas to break down pollutants. But it’s costly. Ferrate should cost less, making it attractive to smaller water-cleanup plants, Reckhow says. Early next year, his mobile water treatment lab plans to test ferrate water treatment in the small town of Gloucester, Mass.
Brian Chaplin is an engineer at the University of Illinois at Chicago. He notes that some water-filtering membranes can get clogged with small particles. Unclogging the filter wastes energy. It also ups the cost of treating water. Electricity might solve that problem, Chaplin suggests, and offer some side benefits.
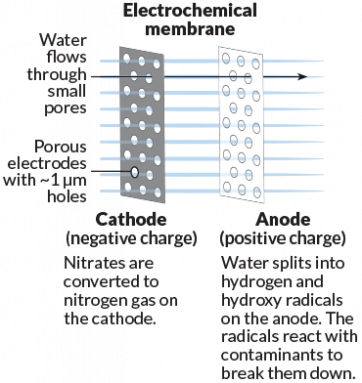
His team tested a special electrically charged membrane made from titanium oxide or titanium dioxide. This electrochemical membrane not only filters water but also acts as an electrode. Chemical reactions happening on such a charged membrane can turn nitrates — a pollutant — into nitrogen gas. Or the membrane might split water molecules, generating reactive ions that can kill infectious microbes in the water. The reactions also prevent particles from sticking to the membrane. Large carbon-based chemicals, such as benzene, now become smaller and less harmful.
In lab tests, these new membranes were successful in filtering out and destroying pollutants, Chaplin says. In one test, a membrane transformed 67 percent of the nitrates into other molecules. The finished water was below the EPA’s regulatory limit for nitrate of 10 parts per million. He and colleagues reported their results last July in Environmental Science and Technology. Chaplin expects to move the membrane into pilot tests within the next two years.
Industrial chemicals known as PFAs present two challenges. Only the larger ones are effectively removed by activated carbon, the filtering substance in many household water filters. Smaller molecules will remain in the water, notes Christopher Higgins. He’s an environmental engineer at the Colorado School of Mines (CSM) in Golden. What’s more, filtering isn’t a simple solution for these pollutants. After all, once removed, they still are hard to break down for safe disposal.
So he and his CSM colleague Timothy Strathmann are working on a process to destroy PFAs. First, they use a specialized filter with tiny holes to grab the molecules out of the water. Then they add sulfite to the concentrated mix of PFAs. When later hit with ultraviolet light, the sulfite generates reactive electrons. These break down the tough carbon-fluorine bonds in the PFA molecules. Within 30 minutes, the UV-sulfites combo almost completely destroyed one type of PFA chemical.
Soon, Higgins and Strathmann will test the process at Peterson Air Force Base in Colorado. It’s one of nearly 200 U.S. sites known to have groundwater tainted by PFAs. Cleaning up those sites would remove the pollutants before they could be used to feed wells or city water systems.