Scientists Say: Dioxide
When two oxygens are attached to some other atom, they get a special name
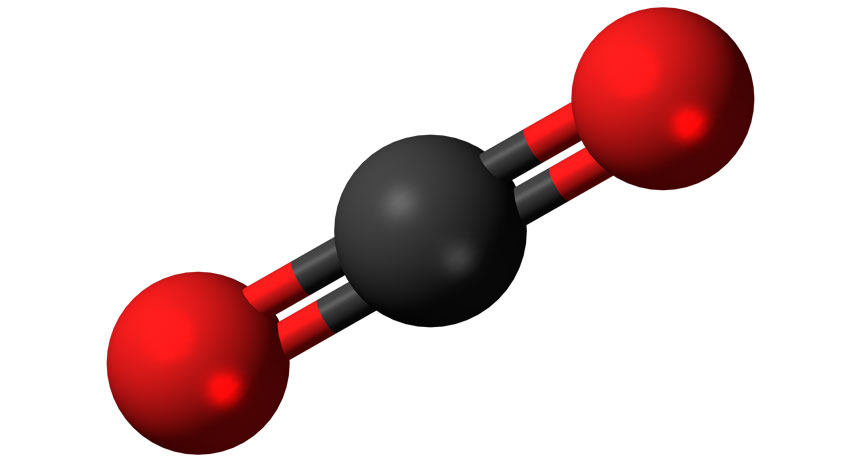
This is a model of a carbon dioxide molecule. The carbon is in black and the two oxygen atoms are in red.
Jynto/Wikimedia Commons
When two oxygens are attached to some other atom, they get a special name

This is a model of a carbon dioxide molecule. The carbon is in black and the two oxygen atoms are in red.
Jynto/Wikimedia Commons