acidic An adjective for materials that contain acid. These materials often are capable of eating away at some minerals such as carbonate, or preventing their formation in the first place.
alkaline An adjective that describes a chemical that produces hydroxide ions (OH-) in a solution. These solutions are also referred to as basic — as in the opposite of acidic — and have a pH above 7.
base (in chemistry) A chemical that produces hydroxide ions (OH-) in a solution. Basic solutions are also referred to as alkaline. (in genetics) A shortened version of the term nucleobase. These bases are building blocks of DNA and RNA molecules.
bleach A dilute form of the liquid, sodium hypochlorite, that is used around the home to lighten and brighten fabrics, to remove stains or to kill germs. Or it can mean to lighten something permanently, such as: Being in constant sunlight bleached most of the rich coloring out of the window drapes.
carbon The chemical element having the atomic number 6. It is the physical basis of all life on Earth. Carbon exists freely as graphite and diamond. It is an important part of coal, limestone and petroleum, and is capable of self-bonding, chemically, to form an enormous number of chemically, biologically and commercially important molecules.
carbon dioxide (or CO2) A colorless, odorless gas produced by all animals when the oxygen they inhale reacts with the carbon-rich foods that they’ve eaten. Carbon dioxide also is released when organic matter burns (including fossil fuels like oil or gas). Carbon dioxide acts as a greenhouse gas, trapping heat in Earth’s atmosphere. Plants convert carbon dioxide into oxygen during photosynthesis, the process they use to make their own food.
climate change Long-term, significant change in the climate of Earth. It can happen naturally or in response to human activities, including the burning of fossil fuels and clearing of forests.
hydrogen The lightest element in the universe. As a gas, it is colorless, odorless and highly flammable. It’s an integral part of many fuels, fats and chemicals that make up living tissues. It’s made of a single proton (which serves as its nucleus) orbited by a single electron.
ion (adj. ionized) An atom or molecule with an electric charge due to the loss or gain of one or more electrons. An ionized gas, or plasma, is where all of the electrons have been separated from their parent atoms.
molecule An electrically neutral group of atoms that represents the smallest possible amount of a chemical compound. Molecules can be made of single types of atoms or of different types. For example, the oxygen in the air is made of two oxygen atoms (O2), but water is made of two hydrogen atoms and one oxygen atom (H2O).
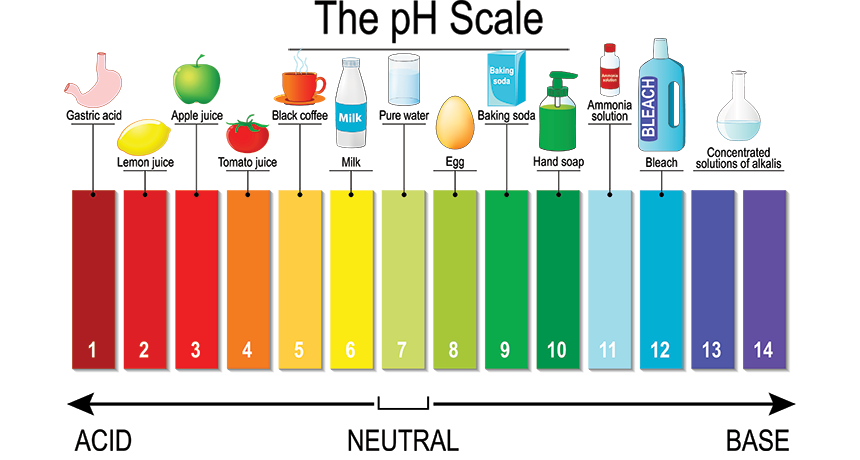
pH A measure of a solution’s acidity or alkalinity. A pH of 7 is perfectly neutral. Acids have a pH lower than 7; the farther from 7, the stronger the acid. Alkaline solutions, called bases, have a pH higher than 7; again, the farther above 7, the stronger the base.
solution A liquid in which one chemical has been dissolved into another.