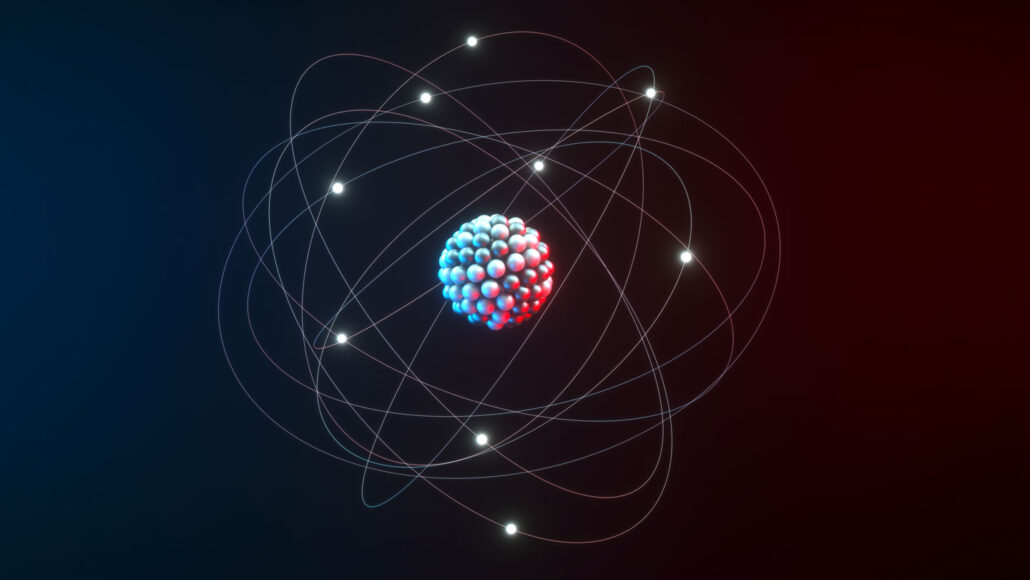
atom: The basic unit of a chemical element. Atoms are made up of a dense nucleus that contains positively charged protons and uncharged neutrons. The nucleus is orbited by a cloud of negatively charged electrons.
bond: (in chemistry) A semi-permanent attachment between atoms — or groups of atoms — in a molecule. It’s formed by an attractive force between the participating atoms. Once bonded, the atoms will work as a unit. To separate the component atoms, energy must be supplied to the molecule as heat or some other type of radiation.
chemical: A substance formed from two or more atoms that unite (bond) in a fixed proportion and structure. For example, water is a chemical made when two hydrogen atoms bond to one oxygen atom. Its chemical formula is H2O. Chemical also can be an adjective to describe properties of materials that are the result of various reactions between different compounds.
chlorine: A chemical element with the scientific symbol Cl. It is sometimes used to kill germs in water. Compounds that contain chlorine are called chlorides.
electron: A negatively charged particle, usually found orbiting the outer regions of an atom; also, the carrier of electricity within solids.
element: A building block of some larger structure. (in chemistry) Each of more than one hundred substances for which the smallest unit of each is a single atom. Examples include hydrogen, oxygen, carbon, lithium and uranium.
neutron: A subatomic particle carrying no electric charge that is one of the basic pieces of matter. Neutrons belong to the family of particles known as hadrons.
nucleus: Plural is nuclei. (in biology) A dense structure present in many cells. Typically a single rounded structure encased within a membrane, the nucleus contains the genetic information. (in astronomy) The rocky body of a comet, sometimes carrying a jacket of ice or frozen gases. (in physics) The central core of an atom, containing most of its mass.
particle: A minute amount of something.
proton: A subatomic particle that is one of the basic building blocks of the atoms that make up matter. Protons belong to the family of particles known as hadrons.
salt: A compound made by combining an acid with a base (in a reaction that also creates water). The ocean contains many different salts — collectively called “sea salt.” Common table salt is a made of sodium and chlorine.
sodium: A soft, silvery metallic element that will interact explosively when added to water. It is also a basic building block of table salt (a molecule of which consists of one atom of sodium and one atom of chlorine: NaCl). It is also found in sea salt.
technology: The application of scientific knowledge for practical purposes, especially in industry — or the devices, processes and systems that result from those efforts.
valence: (in chemistry and physics) The electrons of an atom that are involved in chemical bonding. Valence electrons usually are the outermost electrons (those orbiting farthest from the nucleus).